Download PDF
Cite This Article
Contents
1. Introduction
2. The Common Metabolic Pathway and Chassis Microbes for l -MA Production
3. Metabolic Engineering to Boost the l -MA Production
4. Culture Process Optimization to Increase the Microbial l -MA Production
5. Microbial l -MA Production from Cheap Substrate Utilization
6. Conclusion and Prospects
Author Contributions
Ethics Statement
Informed Consent Statement
Funding
Declaration of competing Interest
References
Current Progress on Microbial l -malic Acid Production
Author Information
Other Information
1
State Key Laboratory of Materials-Oriented Chemical Engineering, College of Biotechnology and Pharmaceutical Engineering, Nanjing Tech University, Nanjing 211800, China
2
Jiangsu Biochemical Chiral Engineering Technology Research Center, Changmao Biochemical Engineering Co., Ltd, Changzhou 213034, China
*
Authors to whom correspondence should be addressed.
Received: 27 March 2024 Accepted: 22 May 2024 Published: 24 May 2024
© 2024 The authors. This is an open access article under the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/).
Synth. Biol. Eng.
2024,
2(2), 10010;
DOI: 10.35534/sbe.2024.10010
ABSTRACT:
As an important
intermediate in the tricarboxylic acid (TCA) cycle, l -malic acid (l -MA)
is also one of the 12 important platform bulk chemicals with high added value.
Owing to its various applications in food, pharmaceuticals, cosmetics and
industry, the global l -MA market
size is growing year by year. Over the last few decades, increasing concerns
regarding fossil fuels depletion and excessive CO2 emissions have
led the global commitment to fostering a green economy and sustainable
development. Alternatively, the sustainable microbial fermentation of l -MA has gradually attracted more and
more attention. Here, this review summarizes the common l -MA biosynthesis pathways and compares the differences
between different chassis microorganisms as well. Moreover, regulation
strategies of genetic metabolic engineering and fermentation process to boost
the l -MA production are
summarized, and the research status of l -MA
production from the cheaper substrates is also discussed. Finally, the
direction of further exploration of industrialized l -MA biosynthesis is proposed, which provides a theoretical
guidance on promoting technological innovation in industrial l -MA production.
Keywords:
l -malic acid; Microbial biosynthesis; Strain engineering; Process optimization
1. Introduction
Malic acid, also known as 2-hydroxysuccinic acid, is a well-known C4 dicarboxylic acid, which has been widely applied in food, pharmaceuticals, oil, wastewater treatment, metal cleaning, textile finishing etc. Due to the asymmetric carbon atoms in the molecular structure of malic acid, there exist two stereoisomers, namely l -malic acid (l -MA) and d -malic acid (d -MA) [1]. However, only the l -MA can be metabolized by living cells [1]. Given the significant commercial value of l -MA, the United States Department of Energy has recognized it as one of the 12 important bulk chemicals with the highest added value. According to a report by IMARC Group, the global malic acid market size reached $211.7 million in 2022, and is expected to reach $283.7 million by 2028, with a compound annual growth rate of 5.1% between 2023 and 2028.
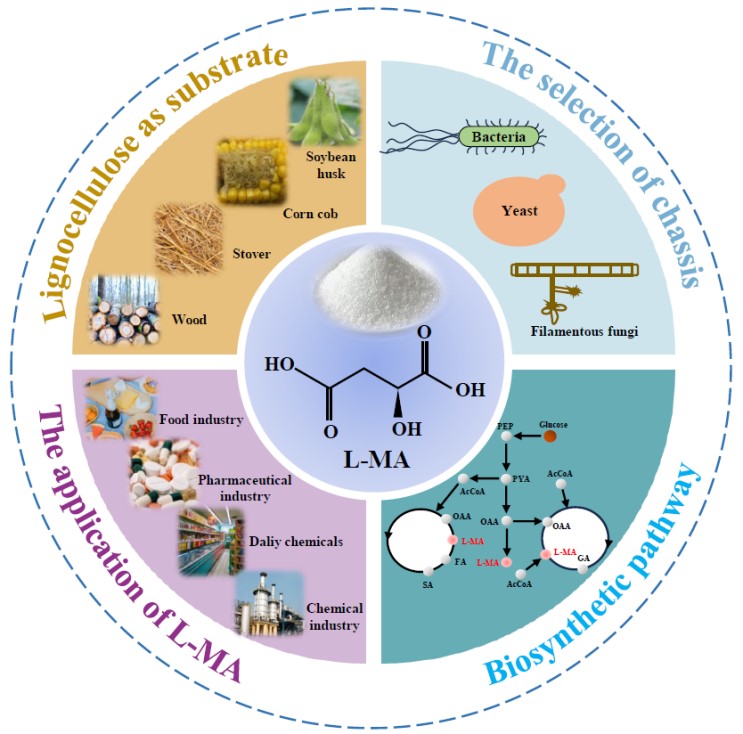
On one hand, natural extraction efficiency of l -MA from immature fruits such as hawthorns, apples, and grapes were quite low (only 0.4~0.7%). On the other hand, chemical synthesis process has the disadvantage of using petroleum-based products and requiring high temperatures and pressures, resulting in severe environmental pollution and excessive carbon emissions [1]. Besides, the final products of chemical synthesis are usually a racemic mixture d,l -malic acid, needing extra separation and extraction [2]. Alternatively, the microbial synthesis provides an environmentally friendly and cost-effective way to synthesize high-purity l -MA. Bacteria, yeasts and filamentous fungi are common chassis for l -MA production due to their characteristics. For example, yeasts are competent in low pH fermentation and environmentally friendly production while filamentous fungi can utilize affordable and abundant lignocellulose to synthesize l -MA (Figure 1).
To better understand the state-of-art of microbial l -MA production, this review will introduce common biosynthesis pathway and chassis microbes for l -MA production. What is more, the differences between different chassis microorganisms will also be compared. Besides, metabolic engineering strategies on boosting l -MA production, such as reinforcement on reductive tricarboxylic acid (rTCA) cycle synthesis pathway, secretion enhancement, by-product reduction and cofactor regulation will be summarized. Furthermore, fermentation process optimization including pH regulation, fed-batch and two-stage strategy will also be concluded. Moreover, the concern on cheap feedstock utilization will also be discussed to save operating costs. Finally, the direction of further exploration in industrial l -MA production will be pointed out.
Figure 1. Panoramic view of microbial production of l -MA.
2. The Common Metabolic Pathway and Chassis Microbes for l -MA Production
2.1. Biosynthesis Pathways of l -MA
Generally, there are three main metabolic pathways for l -MA biosynthesis, namely the tricarboxylic acid (TCA) cycle, the glyoxylic acid route, and the rTCA cycle as Figure 2 shows. TCA cycle begins with oxaloacetate and acetyl-CoA, then citrate synthase converts the substances into citrate. After multiple oxidation and decarboxylation reactions, l -MA was finally formed, accompanied by the release of two carbon dioxide [3]. Thus, a theoretical l -MA yield of TCA cycle was 1 mol/mol glucose. Another l -MA biosynthesis pathway was glyoxylate shunt discovered by Hans Adolf Krebs in 1954, which is a branch pathway of the TCA cycle that enables microorganisms to utilize acetic acid, ethanol, and other C2 substances for growth [4]. Since cyclic glyoxylate shunt completely synthesizes l -MA from acetyl-CoA, its theoretical yield was also 1 mol/mol glucose. Except the two mentioned-above l -MA biosynthesis pathway, another pathway that achieves a theoretical conversion of 2 mol l -MA/mol glucose by fixing CO2 during reaction, namely rTCA cycle. According to a 13C NMR analysis on the typical l -MA producer Aspergillus flavus, it has been confirmed that rTCA pathway was just a simple two-step reaction comprised of pyruvate-oxaloacetate-malate. Specifically, pyruvate carboxylase (pyc) and malate dehydrogenase (mdh) are the two critical enzymes, pyc realized the carboxylation of pyruvate to oxaloacetate, then reduction of oxaloacetate to malate was accomplished under mdh catalysis [2,5]. Especially, this efficient synthesis pathway is located in the cytoplasm, l -MA can be exported into the extracellular more easily than the TCA pathway, which was located in mitochondria. Hence, the rTCA pathway becomes a popular pathway introduced into cell factories for the efficient l -MA production.
2.2. Typical l -MA Producing Microbial Chassis
As reported, although there exist several fungi and yeasts as natural l -MA producers, the performances of these wild types are far from the requirements of industrial production scale. In the meantime, selecting an appropriate starting chassis is the prerequisite to obtain a high-producing strain. Hence, this section describes unique properties of different microorganisms and discusses advantages and disadvantages of the commonly used hosts, providing the references on the efficient l -MA production.
2.2.1. Bacteria
Bacterial l -MA production primarily depended on Escherichia and Bacillus owing to their advantage of clear genetic background, facile genetic manipulation and rapid metabolic rates [6]. However, due to the limited l -MA producing capability, genetic engineering is required. As reported, overexpressing phosphoenolpyruvate carboxykinase (pckA) which mediates the conversion of phosphoenolpyruvate into oxaloacetate can increase oxaloacetate accumulation, contributing to the efficient organic acid production. The strategy also works for the microbial synthesis of l -MA. Soo et al. found that overexpressing gene pckA derived from Mannheimia succiniciproducens in E. coli produced 9.25 g/L of l -MA after 12 h in a 5-L bioreactor. Of course, the appropriate source of pckA is also important, and another engineered E. coli which overexpressed native pckA only produced 1.42 g/L l -MA [7]. To further improve the l -MA production, mdh and pck were co-overexpressed in E. coli BA040 and 13.14 g/L of l -MA with a yield of 0.73 g/g was produced, which obtained an increase of 199.3% and 143.3% than that of the parent one, respectively. What is more, its final l -MA concentration achieved 28.50 g/L with a yield of 0.69 g/g through a scale-up fermentation in 5-L fermentor [8].
2.2.2. Yeasts
Compared to bacteria, several kinds of yeasts are the natural l -MA producers, such as Zygosaccharomyces rouxii, however the amount of l -MA accumulation was still far from the industrial requirements [2]. Picking up a suitable synthesis pathway is important to further improve the l -MA production, and rTCA pathway was the most selected one. For instance, through simultaneously overexpressing pyc2 and mdh3 in Saccharomyces cerevisiae, the final l -MA production was improved to 59 g/L with a productivity of 0.18 g/L/h in a 500-mL flask [9]. Besides, high acid tolerance of yeasts was a prominent advantage for simplifying downstream recycle process, reducing the overall cost and environmental pollution [10,11]. For example, Sun et al. continuously sub-cultured S. cerevisiae under extremely low pH (decreased to pH 2.3) for 1710 generations. After a series of metabolic pathways modifications, the mutant could finally realize 232.9 g/L of l -MA titer with a productivity of 1.62 g/L/h a 3-L fermentor at 144 h without any neutralizer addition [12].
2.2.3. Filamentous Fungi
Compared to the bacteria and yeasts, fungi are a kind of natural l -MA producers, which have a quite high-producing ability. For instance, a typical l -MA producing fungus is A. flavus, whose titer of l -MA reached 113 g/L with a productivity of 0.59 g/L/h after fermentation condition optimization in a 16-L fermentor [13]. However, it was found that A. flavus produced carcinogen aflatoxin during the fermentation process, which is harmful to human health and unsuitable for food-grade l -MA production. Hence, other lower l -MA producing capacity but safer fungi were selected as the starting chassis, such as A. oryzae. To improve its l -MA productivity, native pyc and mdh in A. oryzae NRRL 3488 were overexpressed, improving the l -MA titer from 26.1 g/L to 42.3 g/L with a 28.3% increase [14]. Then, after going through further genetic modifications, A. oryzae NRRL 3488 produced 165 g/L l -MA with a productivity of 1.38 g/L/h in 3-L fed-batch culture, achieving a competitive l -MA productivity [14].
Apart from the advantage of containing native l -MA biosynthetic pathways in some fungal species, fungi have another striking trait of degrading and utilizing cheap substrates. For instance, as an excellent natural lignocellulose degrader, Myceliophthora thermophila produced 181 g/L l -MA from Avicel in a 5-L fed-batch fermentor through co-overexpression of malate transporter (mae) and pyc from A. oryzae [15].
Figure 2. Microbial biosynthetic pathways of l -MA.
3. Metabolic Engineering to Boost the l -MA Production
3.1. Reinforcement on l -MA rTCA Synthesis Pathway
As described above, since rTCA pathway is the most selected one for microbial l -MA biosynthesis owing to its high theoretical yield. Hence, picking up appropriate sources for pyc and mdh is a prerequisite to obtain l -MA hyper-producing strain. Xi et al. combinatorically expressed two source-derived pyc (Pichia kudriavzevii and A. oryzae) and five source-derived cytoplasmic mdh (P. kudriavzevii, Z. rouxii, Corynebacterium glutamicum, S. cerevisiae and A. oryzae) in P. kudriavzevii. And among these ten generating strains, MA006-10 whose pyc and mdh from A. oryzae produced the highest l -MA titer of 43.8 g/L in 250-mL shake [3]. Apart from screening optimal gene sources, selecting proper isozyme also count a lot. For example, cytoplasmic MDH2 was prone to be inactivated by glucose catabolism, while isozyme MDH3 which was derived from the peroxisome has overcome this (MDH3ΔSKL). Hence, overexpressed MDH3ΔSKL in S. cerevisiae has a higher MDH activity, achieving 4.2 g/L l -MA titer with a 3.6-fold increase [12]. Apart from exploring malate dehydrogenase with higher activity, inactivation enzymes which is harmful to it also counts. For example, glucose-induced degradation defective (GID) complex facilitated the degradation of MDH2, which is detrimental to l -MA synthesis. And the missense mutation in GID4 subunit in S. cerevisiae resulted in the inactivation of GID complex, contributing to a 14.2-fold increase in l -MA production [16].
3.2. Secretion Enhancement
In spite of introducing pivotal genes to promote l -MA generation, strengthening the cytoplasmic export of l -MA into the external by overexpressing transporter genes also have a significant effect on l -MA productivity. For example, by introducing a malate transporter gene SpMAE1 derived from Schizosaccharomyces pombe to an acid-tolerant yeast P. kudriavzevii, the l -MA titer was improved to 43.8 g/L from below 3 g/L in 250-mL flask [3]. Similarly, Sun et al. verified that a SpMae variant enhanced the export capacity of l -MA successfully, contributing to 59.9 g/L l -MA with a 25% increase and a reduction of fermentation time by 72 h in S. cerevisiae [12]. Apart from affecting l -MA production in yeasts, introduction of carboxylic acid transporters also works in fungi. For instance, overexpressing the native C4-dicarboxylic acid transporter in A. oryzae NRRL 3488 improved l -MA concentrations to 60 g/L, roughly two-fold of that of wild-type (27 g/L) after 72 h [5]. Moreover, simultaneous overexpression of A. oryzae-derived C4-dicarboxylate transporter gene and S. pombe-derived l -MA permease gene improved the l -MA titer of A. oryzae from 58.5 g/L to 89.5 g/L [14].
3.3. By-products Reduction
For efficient l -MA production, enhancing carbon flux to target products is not enough, it is also requisite to suppress the shunt of the by-product formation to reduce competition on carbon metabolic flow. Besides, by-products mixture brought out more difficulty in downstream separation and extraction. As reported, the most accumulated by-product during l -MA biosynthesis are fumaric acid, succinic acid, lactic acid, formic acid, citric acid as well as acetic acid [2].
Specifically, gene fumB (coding fumarase), frdABCD (coding fumarate reductase), ldhA (coding lactate dehydrogenase), pflB (coding pyruvate formate-lyase) and poxB (coding pyruvate oxidase) was respectively related to fumaric acid, succinic acid, lactic acid, formic acid and acetic acid biosynthesis. Thus, destruction of these by-product generating pathway may boost the l -MA production. For example, Dong et al. sequentially knocked out frdBC, fumB and fumAC in E. coli F0511 in order to inhibit the conversion of l -MA to succinate, resulting in succinate accumulation was decreased by 64.9%, 76.9%, and 91.5%, respectively compared to the parent one. Accordingly, the highest l -MA titer was increased up to 7.78 g/L [17].
3.4. Cofactor Regulation
In addition to the strategies mentioned above, cofactor regulation could be considered as another working strategy on l -MA production improvement. For example, introducing soluble pyridine nucleotide transhydrogenase SthA derived from E. coli (EcSthA) into engineered P. kudriavzevii successfully improved l -MA production to 38.3 g/L in 250-mL flask. The underlying mechanism was that EcSthA provided adequate cytosolic NADH, which is important to activate malate dehydrogenase and the by-product pyruvic acid accumulation was decreased to 2.9 g/L from 7.9 g/L at the same time [3]. Similarly, overexpressing of NADH oxidase LlNOX from Lactobacillus lactis leads to a 36.7% decrease on succinic acid unexpectedly [18]. Aside from NADH content regulation, cofactor NADPH also has an effect on l -MA improvement. For instance, Dong et al. introduced gene pos5 coding NADH kinase into E. coli W3110 to provide more NADPH, and this engineered E. coli W3110 produced 21.65 g/L l -MA with a yield of 0.36 g/g in a 5-L bioreactor with a 183.4% increase [17].
4. Culture Process Optimization to Increase the Microbial l -MA Production
4.1. pH Regulation
It is well-known that fermentation conditions play key roles in l -MA production, especially pH. Generally, near-neutral pH (6.0–6.5) was considered as optimal pH for effective l -MA production, no matter for bacteria, yeasts and fungi [3,9]. However, the medium pH decreases markedly due to the overproduction of acidic products, which severely inhibits the metabolic activity and the production of cells [12]. Zambanini et al. found that dissolved l -MA in the medium does cause a product inhibition as soon as the concentration reached 100 g/L and addition of neutralizing agent to the broth precipitated out the l -MA into carbonate form, greatly alleviating product inhibition and toxicity on strain [19]. And among various kinds of neutralizers, CaCO3 is the most used one. A study has proved that l -MA titer reached 129 g/L under the CaCO3-buffered system while the titer just reached 4.0 g/L in MES-buffered system [20]. The reason is that CaCO3 not only has the ability of maintaining fermentation broth at appropriate pH, but also supplies the CO2 required for the l -MA production. For instance, enhanced l -MA titer of 195 g/L and productivity of 0.74 g/L/h was achieved in Ustilago trichophora TZ1 in a 2.5-L bioreactor with CaCO3 buffer system, while the titer and productivity of the initial one was 142 g/L and 0.54 g/L/h, respectively [20]. What is more, the addition amount of neutralizer also accounts. For example, a modified yeast P. kudriavzevii produced only 53.2 g/L l -MA with 20 g/L CaCO3 addition, whereas 199.4 g/L of l -MA with a yield of 0.94 g/g was achieved in a 3-L fermentor when CaCO3 neutralizer increased to 70 g/L [3].
4.2. Fed-batch Strategy
Another major limitation of organic acid production during fermentation is the substrate inhibition, especially when using glucose as the substrate. Hence, fed-batch strategy is a commonly adopted method. For example, the maximum l -MA titer reached 165 g/L at 120 h in a 3-L fed-batch fermentor by an engineered A. oryzae strain, which was increased by 17.9% and 13.1% compared with that in 250-mL shake flasks and in batch culture, respectively [14]. Similarly, an engineered Trichoderma reesei produced 220.5 g/L l -MA with the productivity of 1.15 g/L/h in a 5-L fed-batch culture while only produced 105 g/L l -MA in 250-mL flask culture [21].
4.3. Two-stage Regulation Strategy
The two-stage regulation strategy was another commonly used strategy to maintain a balance between microbial growth and metabolite formation, bringing in higher target titer. The underlying mechanism of two-stage strategy is that aerobic phase supported growth of the strain, while anaerobic phase was designed to minimize the carbon flux to biomass while maximize the carbon flow to the target products. For example, Zhang et al. adapted a two-stage strategy during l -MA fermentation by E. coli, namely cells grown aerobically (1.0 vvm air) for 16 h and then shifted to anaerobic conditions for l -MA production (72 h). With this approach, 33.9 g/L l -MA was produced with a yield of 1.42 mol/mol glucose in 3-L bioreactor, higher than 21.9 g/L of l -MA without two-stage regulation [22]. Similarly, Jiang et al. conducted a dual-phase fermentation process of the recombinant E. coli BA063 for higher l -MA titer. Specifically, aerobic culture was finished when DCW reached around 11 g/L, then shifted into anaerobic phase by sparging CO2. Finally, l -MA concentration of this engineered E. coli reached 28.50 g/L with a yield of 0.69 g/g within 67 h in a 5-L fermentor [8]. Besides, anaerobic condition also had an effect on ATP level and intracellular NAD(H) pool enhancement, resulting in l -MA yield increase.
5. Microbial l -MA Production from Cheap Substrate Utilization
5.1. Utilization of Glycerol or Methanol as the Feedback
From the above sections, it can be deduced that glucose is the most widely used fermentable substrates in microbial l -MA production cases. However, to further meet the industrial interests, cheaper carbon sources need exploring. One of potential carbon sources is crude glycerol, a major by-product accounting for approximately 10% of the total volume of produced biodiesel [23]. To make use of this kind of feedstock for l -MA production, Zambanini et al. obtained the U. trichophora RK089 which could synthesize l -MA through glycerol assimilation after screening 74 Ustilago strains [19]. And further adaptive laboratory evolution (ALE) improved glycerol intake and l -MA synthesis efficiency, the growth and production rate of U. trichophora TZ1 reached 0.26 g/L/h and 3.5 g/L/h, which were increased by 2.5- and 6.6-fold, respectively after domestication. Along with further medium optimization, the final titer of l -MA reached 196 g/L in 500-mL flask [20]. Apart from crude glycerol, methanol is another promising feedstock owing to its low price, abundance, energy richness and renewability [24]. Similar to the strategy above-mentioned, A. niger MTCC 281 was sub-cultured adaptively in the medium containing methanol (1–5%) and l -MA (40–80 g/L) for 22 weeks. Finally, with enhanced stress tolerance, the mutant A. niger MTCC produced 62.54 g/L of l -MA after 192 h, 4.45-fold higher than the control strain MTCC 281 [25].
5.2. Utilization of Lignocellulose as the Feedback
Lignocellulose is a kind of abundant and renewable resource, but the complex structure hinders its utilization [26]. Thus, the pretreatment on lignocellulosic substrates is commonly required [27,28]. However, as one of the major components, the poor utilization capability of xylose usually hinders the efficient total component sugars utilization of lignocellulose hydrolysate [29]. For example, S. cerevisiae CTMAE produced 70 mg l -MA/g xylose under 10 g/L xylose condition after the XR-XDH assimilation pathway import in 125-mL flask [30]. Furthermore, by eliminating the by-product pathway, enhancing the l -MA synthesis pathway, transport function and a fed-batch fermentation, l -MA titer of S. cerevisiae CTMAE reached 61.2 g/L with a yield of 0.23 g/g xylose in a 3-L bioreactor [31]. However, a problem worth noting is that lignocellulose hydrolysate usually contains quite amounts of inhibitors, such as furans, weak acids and phenolic aldehydes, having a negative effect on cell growth and product generation. Alternatively, some fungi with the strong cellulose degradation ability, becoming the promising chassis microorganisms. For example, after expressing an exogenous pyc derived from C. glutamicum ATCC 13032, 62.76 g/L of l -MA was directly produced from 100 g/L cellulose by a modified strain Thermobifida fusca muC in 3-L fermentor. What is more, it even could directly degrade milled corn stover and obtain 21.47 g/L of l -MA [32].
6. Conclusion and Prospects
With the expanding demand for l -MA, more and more attentions were paid to realize biological production for its environmental protection. And selecting an appropriate starting chassis is the fundamental step. Based on this review, it can be deduced that bacteria have a relatively low l -MA titer than that of the yeasts and fungi as Table 1 shows. What is more, yeasts have an obvious advantage of significant acid-tolerance capacity, contributing to reduced neutralizer addition and lower investment cost. When it comes to fungi, which has a native ability of utilizing cheap lignocellulose feedbacks, saving overall cost as well. Collectively, yeasts and fungi chassis exhibit superior performance in l -MA production compared to bacterial ones.
Besides, it is also worth noting that filamentous fungal has another unique feature, namely its specific morphological is closely correlated with target production [14,33]. Hence, making full use of this trait is also a direction for l -MA production enhancement. For example, Chen et al. realized 142.5 g/L l -MA of A. oryzae in a 7.5-L fermentor, much higher than 105.5 g/L of the origin one through overexpression of gene cdc14 (cell division cycle 14) with three copies [33]. Similarly, deletion of gul1 which encodes a putative mRNA-binding protein related to hyphal morphology in T. reesei boosting the l -MA production reached 235.8 g/L from 170 g/L in 5-L fermentor [34].
Apart from breeding high-producing chassis through metabolic engineering, there still exist another three problems as for l -MA microbial fermentation process. One is a plenty of neutralizers should be added to maintain the neutral pH, supporting microorganism growth and organic acid generation in its carboxylate form. Consequently, inorganic acids sulfuric or hydrochloric acid was added to release carboxylic acids form, which bring the challenges for the downstream extraction process, such as the increased environmental pollution and overall production costs. Hence, realizing reduction on neutralizers usage is a main requirement especially when it comes to industrial production. And ALE is a promising strategy to breed acid-tolerant strains, making for neutralizers decrease. Besides, further genetic modifications could focus on membrane engineering to enhance microbial resistance to stress.
The other problem need solving is the difficulty in downstream separation and extraction process. As reported, the emerging innovation extraction techniques, namely in-situ product recovery (ISPR) system by trioctylamine in 1-octanol successfully improved l -MA titer of A. niger PJR1 to 131.48 g/L from 115.67 g/L through fed-batch extractive fermentation [35]. This approach decreases the adverse effects of stress caused by low pH and high l -MA concentrations on the physiological traits of the producing strain and simplified downstream process.
Last but not least, the costs of substrates should be reduced. Although the lignocellulose hydrolysate is considered as a promising substrate, the inhibitors hinder the cell growth and the final l -MA production. Hence, approaches towards elimination of inhibitors should be developed. As reported, detoxification treatment is a promising strategy [32]. Besides, ALE to improve the strain tolerance, genetic modifications on resistance ability enhancement both can be considered as promising strategies to overcome the inhibitor problem. Collectively, to further promote l -MA industrial production scale, ideal l -MA cell factories with high productivity and strong resistance ability need breeding through integrating disciplines. Furthermore, in conjunction with innovative separation and extraction techniques downstream process, the cost will be further optimized.
Author Contributions
Conceptualization, M.J. and Y.J.; Validation, W.J., W.Z., and F.X.; Writing—Original Draft Preparation, L.M.; Writing—Review & Editing, L.M. and M.Q.; Supervision, Y.J.; Project Administration, M.J.
Ethics Statement
Not applicable.
Informed Consent Statement
Not applicable.
Funding
This research was funded by National Key R & D Program of China (2022YFC3401301), Shandong Taishan Industrial Experts Program (202306155), Open Funding Project of Key Laboratory for Crop and Animal Integrated Farming and State Key Laboratory of Materials-Oriented Chemical Engineering (No. KL-MCE-23A10), China Postdoctoral Science Foundation (No. 2023M740370).
Declaration of competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1.
Wu N, Zhang JH, Chen YR, Xu Q, Song P, Li Y, et al. Recent advances in microbial production of L-malic acid. Appl. Microb. Biotechnol. 2022, 106, 7973–7992. [Google Scholar]
2.
Xi Y, Fan F, Zhang X. Microbial l-malic acid production:
History, current progress, and perspectives. Green Carbon. 2023, 1, 118–132. [Google Scholar]
3.
Xi Y, Xu H, Zhan T, Qin Y, Fan F, Zhang X. Metabolic engineering of the acid-tolerant yeast Pichia kudriavzevii for efficient L-malic acid production at low pH. Metab. Eng. 2023, 75, 170–180. [Google Scholar]
4.
Kornberg HL, Krebs HA. Synthesis of Cell Constituents from C2-Units by a Modified Tricarboxylic Acid Cycle. Nature 1957, 179, 988–991. [Google Scholar]
5.
Brown SH, Bashkirova L, Berka R, Chandler T, Doty T, McCall K, et al. Metabolic engineering of Aspergillus oryzae NRRL 3488 for increased production of l-malic acid. Appl. Microb. Biotechnol. 2013, 97, 8903–8912. [Google Scholar]
6.
Yang D, Prabowo CPS, Eun H, Park SY, Cho IJ, Jiao S, et al. Escherichia coli as a platform microbial host for systems metabolic engineering. Microb. Cell Fact. Book 2021, 65, 225–246. [Google Scholar]
7.
Moon SY, Hong SH, Kim TY, Lee SY. Metabolic engineering of Escherichia coli for the production of malic acid. Biochem. Eng. J. 2008, 40, 312–320. [Google Scholar]
8.
Jiang Y, Zheng T, Ye X, Xin F, Zhang W, Dong W, et al. Metabolic engineering of Escherichia coli for l-malate production anaerobically. Microb. Cell Fact. 2020, 19, 165. [Google Scholar]
9.
Zelle RM, de Hulster E, van Winden WA, De Waard P, Dijkema C, Winkler AA, et al. Malic acid production by Saccharomyces cerevisiae: Engineering of pyruvate carboxylation, oxaloacetate reduction, and malate export. Appl. Environ. Microb. 2008, 74, 2766–2777. [Google Scholar]
10.
Liu TT, Sun L, Zhang C, Liu Y, Li J, Du G, et al. Combinatorial metabolic engineering and process optimization enables highly efficient production of L-lactic acid by acid-tolerant Saccharomyces cerevisiae. Bioresour. Technol. 2023, 379, 129023. [Google Scholar]
11.
Malubhoy Z, Bahia FM, de Valk SC, de Hulster E, Rendulić T, Ortiz JPR, et al. Carbon dioxide fixation via production of succinic acid from glycerol in engineered Saccharomyces cerevisiae. Microb. Cell Fact. 2022, 21, 102. [Google Scholar]
12.
Sun L, Zhang QW, Kong X, Liu Y, Li J, Du G, et al. Highly efficient neutralizer-free L-malic acid production using engineered Saccharomyces cerevisiae. Bioresour. Technol. 2023, 370, 128580. [Google Scholar]
13.
Battat E, Peleg Y, Bercovitz A, Rokem JS, Goldberg I. Optimization of L-malic acid production by Aspergillus flavus in a stirred fermentor. Biotech. Bioeng. 1991, 37, 1108–1116. [Google Scholar]
14.
Liu J, Xie Z, Shin H-D, Li J, Du G, Chen J, et al. Rewiring the reductive tricarboxylic acid pathway and L-malate transport pathway of Aspergillus oryzae for overproduction of L-malate. J. Biotech. 2017, 253 , 1–9. [Google Scholar]
15.
Li JG, Lin LC, Sun T, Xu J, Ji J, Liu Q, et al. Direct production of commodity chemicals from lignocellulose using Myceliophthora thermophila. Metab. Eng. 2020, 61 , 416–426. [Google Scholar]
16.
Negoro H, Matsumura K, Matsuda F, Shimizu H, Hata Y, Ishida H. Effects of mutations of GID protein-coding genes on malate production and enzyme expression profiles in Saccharomyces cerevisiae. Appl. Microb. Biotech. 2020, 104, 4971–4983. [Google Scholar]
17.
Dong XX, Chen XL, Qian YY, Wang Y, Wang L, Qiao W, et al. Metabolic engineering of Escherichia coli W3110 to produce L-malate. Biotech. Bioeng. 2017, 114, 656–664. [Google Scholar]
18.
Liu JJ, Li JH, Liu YF, Shin HD, Ledesma-Amaro R, Du G, et al. Synergistic Rewiring of Carbon Metabolism and Redox Metabolism in Cytoplasm and Mitochondria of Aspergillus oryzae for Increased L-Malate Production. Acs Synth Biol. 2018, 7, 2139–2147. [Google Scholar]
19.
Zambanini T, Sarikaya E, Kleineberg W, Buescher JM, Meurer G, Wierckx N, et al. Efficient malic acid production from glycerol with Ustilago trichophora TZ1. Biotech. Biof. 2016, 9, 67. [Google Scholar]
20.
Zambanini T, Kleineberg W, Sarikaya E, Buescher JM, Meurer G, Wierckx N, et al. Enhanced malic acid production from glycerol with high-cell density Ustilago trichophora TZ1 cultivations. Biotech. Biof. 2016, 9, 1–10. [Google Scholar]
21.
Chen YM, Han A, Wang M, Wei D, Wang W. Metabolic Engineering of Trichoderma reesei for L-Malic Acid Production. J. Agr. Food Chem. 2023, 71, 4043–4050. [Google Scholar]
22.
Zhang X, Wang X, Shanmugam KT, Ingram L. L-Malate Production by Metabolically Engineered Escherichia coli. Appl. Environ. Microb. 2011, 77, 427–434. [Google Scholar]
23.
Kong PS, Aroua MK, Daud WMAW. Conversion of crude and pure glycerol into derivatives: A feasibility evaluation. Renew. Sus. Energy Rev. 2016, , 63 , 533–555. [Google Scholar]
24.
Lee EY, Sarwar A. Methanol-tolerant yeast for biofuel production. Nat. Metab. 2022, 4, 800–801. [Google Scholar]
25.
Iyyappan J, Bharathiraja B, Baskar G, Jayamuthunagai J, Barathkumar S. Malic acid production by chemically induced Aspergillus niger MTCC 281 mutant from crude glycerol. Bioresour. Tech. 2018, 251 , 264–267. [Google Scholar]
26.
Deng Y, Mao Y, Zhang X. Metabolic engineering of a laboratory-evolved Thermobifida fusca muC strain for malic acid production on cellulose and minimal treated lignocellulosic biomass. Biotech. Progr. 2016, 32, 14–20. [Google Scholar]
27.
Xia J, Xu JX, Hu L, Liu X. Enhanced poly(L-malic acid) production from pretreated cane molasses by Aureobasidium pullulans in fed-batch fermentation. Prep. Biochem. Biotech. 2016, 46, 798–802. [Google Scholar]
28.
Cheng C, Zhou YP, Lin M, Wei P, Yang ST. Polymalic acid fermentation by Aureobasidium pullulans for malic acid production from soybean hull and soy molasses: Fermentation kinetics and economic analysis. Biores. Tech. 2017, 223 , 166–174. [Google Scholar]
29.
Carroll A, Somerville C. Cellulosic Biofuels. Annu. Rev. Plant Biol. 2009, 60, 165–182. [Google Scholar]
30.
Kang NK, Lee JW, Ort DR, Jin YS. L-malic acid production from xylose by engineered Saccharomyces cerevisiae. Biotech. J. 2022, 17, 2000431. [Google Scholar]
31.
Kim SR, Park YC, Jin YS, Seo JH. Strain engineering of Saccharomyces cerevisiae for enhanced xylose metabolism. Biotech. Adv. 2013, 31, 851–861. [Google Scholar]
32.
Xia J, Qiu ZY, Ma SB, Liu Q, Han R, Liu X, et al. Efficient polymalic acid production from corn straw hydrolysate by detoxification of phenolic inhibitors. Front. Bioeng. Biotech. 2023, 11, 1339982. [Google Scholar]
33.
Chen XL, Zhou J, Ding Q, Luo Q, Liu L. Morphology engineering of Aspergillus oryzae for l-malate production. Biotech. Bioeng. 2019, 116, 2662–2673. [Google Scholar]
34.
Chen YM, Wang JJ, Wang M, Luo Q, Liu L. Engineering the metabolism and morphology of the filamentous fungus Trichoderma reesei for efficient L-malic acid production. Biores. Tech. 2023, 387, 2662–2673. [Google Scholar]
35.
Iyyappan J, Baskar G, Bharathiraja B, Gopinath M. Enhanced malic acid production using Aspergillus niger coupled with in situ product recovery. Biores. Tech. 2020, 308, 123259. [Google Scholar]
36.
Mu L, Wen JP. Engineered Bacillus subtilis 168 produces l-malate by heterologous biosynthesis pathway construction and lactate dehydrogenase deletion. World J. Microb. Biot. 2013, 29, 33–41. [Google Scholar]