Download PDF
Cite This Article
Contents
1. Introduction
2. Natural Fibers
3. Biodegradable Polymers
4. Green Composites/Bio-composites
5. Processing Techniques for Preparation of Biocomposites
6. Characterization of Biocomposites
7. Applications of Biocomposites
8. Current Challenges for Biocomposites
9. Future Scope of Biocomposites
10. Conclusions
Acknowledgement
Author Contributions
Funding
Declaration of Competing Interest
List of Abbreviations
References
Green Composites Using Naturally Occurring Fibers: A Comprehensive Review
Author Information
Other Information
School of Material Science and Technology, Indian Institute of Technology (Banaras Hindu University), Varanasi 221005, India
*
Authors to whom correspondence should be addressed.
Received: 01 June 2023 Accepted: 09 August 2023 Published: 15 August 2023
© 2023 The authors. This is an open access article under the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/).
Sustain. Polym. Energy
2023,
1(2), 10010;
DOI: 10.35534/spe.2023.10010
ABSTRACT:
Depletion of non-renewable resources and health hazards of petroleum-based polymers and plastics has enforced the development of eco-friendly materials. The use of conventional plastics has to be minimized and can be replaced with environmentally friendly and sustainable bio-based polymers or biopolymers due to extensive environmental impact. A major share of petroleum-based polymers is used for polymeric composites with research focus on green composites and biocomposites containing renewable/bioderived matrix polymer and fillers from naturally occurring fibers. Biocomposites hold great promise to replace petroleum-based polymer composites owing to their lower cost, non-toxicity, abundance of raw material, renewability, and high specific strength. All these merits of biocomposites have led to an increment in the development of new biocomposites with enhanced properties, wide applicability and ever demanding criteria. The recently published review studies detailed the raw materials used, fabrication techniques, characterization, and applications including biodegradation and rheological studies performed in recent years. This review covers all the important properties of biocomposites along with detailed description of synthesis, properties, characterizations and applicability of these green composites in several areas. The review also focuses on their raw materials, types, recent biocomposites, processing techniques, characterizations, applications, and current challenges with future aspects.
Keywords:
Biopolymers; Natural fibers; Biocomposites; Biodegradation; Sustainability
1. Introduction
Polymer-based composites are an important class of materials used in every field such as medicine, automotive, construction, household, textiles, and aviation sectors [1]. Most of the polymers used for composites are petroleum based non-renewable and non-biodegradable which pollute the environment along with affecting every sort of life present on earth. Growing demand for polymeric composite materials in various sectors is one of the reasons for uncontrolled manufacturing and extensive use of petroleum-based polymers and synthetic fibers which results in faster depletion of valuable non-renewable resources, increase in health issues [2], and pollution [3]. Rising environmental concerns, strict restrictions on nondegradable plastic use, higher cost and energy consumption for the production of synthetic fibers, health issues, and demand for sustainability have attracted attention toward environment-friendly and sustainable polymeric composites [4]. Since the last three decades, the use of biopolymers has been increasing for the development of sustainable products and to minimize the use of petroleum-based polymers [5]. These materials could easily solve the non-degradability and non-sustainability issues of petroleum-based polymers and their use in polymeric composites [6]. To deal with the problems of conventional polymeric composites, researchers have shifted their studies towards “green composites or biocomposites” which are completely made up of biodegradable and renewable natural resources [7]. Biocomposites or green composites are the composites made up of biopolymers (degradable or made from renewable resources) as matrix and natural fibers/fillers as reinforcement [8]. They are not new for the scientific community now as they are being used for ages. Before the discovery of petroleum-based polymers, almost all kinds of composites used were made up of naturally occurring renewable materials such as rice straws and clay mixture air-dried bricks to prepare homes which is the oldest use of composites. Mesopotamians used glued wood strips to fabricate plywood around 3400 B.C. [9]. The use of petroleum-based plastics for composites or modern composites started in 1905 when the first plastic and asbestos fiber-based composite was fabricated by Belgian chemist Leo Baekeland.
The mid-1900s was the start of the boom in modern composites after which biomaterials-based composites were forgotten [10]. However, after the 1990s increased attention has been given to biopolymers, natural fibers, and their composites due to growing awareness of the impact of extensive use of nondegradable polymers and synthetic fibers [11]. Biocomposites can easily be recycled and disposed of after their use without damaging the environment. A recent survey on the market growth of biocomposites shows that during the years 2016 to 2024, the global market size for biocomposites is increasing at a compound annual growth rate of 11.8%. In 2016 the market value of biocomposites was 4.46 billion USD which is expected to become 10.89 billion USD by 2024. The use of biomaterials in material fabrication has increased from 5% in 2004 to 12% in 2010 and is expected to become 25% by 2030 [12]. Biocomposites have advanced considerably in terms of processing, characterizations, and use of various new materials and applications [13]. Natural fibers such as hemp, bamboo, ramie, kenaf, flax, and jute have gained significant focus as potential replacements for synthetic fibers like glass and carbon [14]. Biopolymers such as poly(lactic acid), cellulose, starch, and synthetic polymers which are completely biodegradable can be used as the matrix to fabricate biodegradable composites [15]. Hundreds of studies have been performed to investigate the mechanical, thermal, biodegradability, and biocompatibility of biocomposites made of biopolymers such as poly (lactic acid) (PLA), polyhydroxy alkanoates (PHAs), etc., and natural fibers. The characteristics which make biocomposites as better substitute are thermal insulation, CO2 neutrality, specific mechanical properties, good damping behaviour, high acoustic insulation, low density, availability of raw material, high health safety, non-toxic, less production energy, and lightweight [16]. All these merits of biocomposites have attracted a lot of research attention which is continuously increasing every year resulting in the betterment of processing techniques and other pre-treatments of fibers and polymers before fabrications of biocomposites. This increasing research work in last two decades gave biocomposites with better properties, and hence their applicability and demand are increasing day by day. Especially, the European countries have been using natural fibers in the automotive sector since the 1990s and leading all over the world for the use of biocomposites and green composites. In the last few years, papers about biocomposites and green composites have also increased manyfold. A review with a detailed description of biodegradation of biocomposites and rheological analysis was required to cover these necessary aspects of biocomposite studies. This review includes recent works on biocomposites, biomaterials to fabricate biocomposites, and fabrication/processing techniques. Characterization techniques for mechanical, thermal, morphological, and chemical properties are included. The biodegradation tests and rheological studies of the biocomposites have been discussed in the characterization section of the review article with examples. The characterization section is followed by the application of biocomposites with recent examples along with current challenges and possible future scopes discussed in the next section.
2. Natural Fibers
Natural fibers as filler for composite materials are a potential alternative to synthetic fibers, because of their flexibility, environmental friendliness, lower cost, renewability, and abundance all through the globe [17]. The plants from which these fibers are extracted are renewable and sustainable, but not the plant fibers itself [18]. In recent years, increasing research efforts have been given to enhance the utilization of plant-based natural fibers, such as hemp, jute, bamboo, kenaf, ramie, and pineapple leaf fibers due to environmental and sustainability concerns [19]. Natural fibers have been utilized to make ropes, twines, fabrics, mud bricks for construction and much more, therefore, playing a significant role in the society. Natural fibers do not pollute the environment as they are fully biodegradable, natural, and carbon neutral [20]. Due to their versatility and local availability, natural fibers are potential alternative materials to synthetic fibers in the composite industry [21]. In recent years, natural fibers are preferred over synthetic fibers because of their easy processing as they produce negligible wear and tear to machines and parts during processing, and are not healthily hazardous such as glass fiber [22]. The exceptional strength per unit mass of natural fiber makes them an excellent and attractive choice as reinforcing materials [23]. According to DNFI organization, 33.7 million tons of natural fibers are produced in 2022 and are used for various applications like fabrics, paper production, packaging, sports equipment, automobiles, and building materials [24]. Natural fibers can be used instead of synthetic fibers to make fiber-reinforced polymer composites [25]. Natural fiber reinforced composites are applied in various sectors like aerospace, automotive, and construction because of their higher strength, stiffness, availability, lightweight, and biodegradability [26]. The specific strength (strength to weight ratio) and specific stiffness (stiffness to weight ratio) of natural fibers are several folds larger than aluminium and steel which cannot be achieved by metals, ceramics, and polymers alone [27]. Natural fibers are found abundantly in nature and can be extracted from plants, animals, and minerals [28]. For plant fibers, their diameter and the surface properties vary with processing methods, size, type, and maturity of the plant. Mechanical, physical, and chemical properties depend upon the structure and chemical composition of the plant [29]. Excellent mechanical properties such as hemp, sisal, bamboo, coconut, and jute are used as reinforcement in these biopolymer matrices. Table 1 displays some natural fibers along with their mechanical properties. There are hundreds of types of plants from which can be used to extract fibers but only some of them are mostly used because of their better mechanical properties. Other than plants, animals and minerals are also used to obtain fibers which are presented in next section for the classification of natural fibers.2.1. Classification of Natural Fibers
Based on their origin, natural fibers are classified as animal, plant, and mineral fibers. Mostly, animal fibers are composed of proteins while plant fibers are composed of cellulose, whereas the mineral fibers are made up of natural minerals. Plant fibers are abundantly available and cost-effective; on the other hand, animal fibers have recently gained attention as reinforcing material for biopolymer composites. Plant fibers are mostly used to make green composites as plant fibers are abundant in nature, do not depend on animals, and do not affect health like asbestos fibers. The classification of fibers is shown in Figure 1. In most of the cases, these fibers are modified on the surface before using in the biocomposites. Chemical treatments are the most prominent one for which a detailed description is given in the subsequent section.2.2. Chemical Treatments of Natural Fibers
Natural fibers have some innate drawbacks such as irregularity in the size, shape, and diameter of the fiber, and their effect on the density, hardness, and strength of the composite can be observed [20]. Other major problems of natural fibers are poor resistance to microbes and pests, high moisture sensitivity, and moderate thermal stability [28]. The high hydrophobicity of natural fibers obstructs widening their reinforcement in composites in automotive applications. The function of natural fibers as compliance products are affected by the hydroxyl (-OH) and other polar groups and various physical impurities found in the fibers [19]. The unpredictable durability affecting both the processing and application of natural fibers is a major obstacle of using them in composites [29]. To overcome these shortcomings, the natural fibers are subjected to biological, physical, and chemical treatments to remove impurities and improve water resistance, mechanical properties, and interfacial bonding with the composite matrix. Pannu and his team [54] obtained a 34% increment in tensile strength of banana fiber-reinforced Polylactic Acid (PLA) composites after treating fibers with sodium hydroxide (NaOH), i.e., alkaline treatment. Another study performed by Lo Re et al. used acetylated and untreated cellulose fibers to prepare wood cellulose fiber reinforced poly(ε-caprolactone) biocomposites. An increment in ultimate strength and Young’s modulus by 46% and 248%, respectively, were obtained when compared to untreated fibers reinforced composites [55]. Table 2 lists some more studies on the chemical modification of natural fibers.
Table 1. Various plant fibers with their mechanical properties.
Figure 1. Classification of natural fibers.
Table 2. Chemical treatments and changes in the natural fibers after treatment.
3. Biodegradable Polymers
Biodegradable polymers, derived from nature or by synthetic route, can be broken down by enzymes from the biosphere at proper pH and temperature environment [73]. Biodegradable polymers that are obtained from plants, animals, and microbes are considered as biopolymers [74]. They are abundantly available renewable materials usually employed to produce eco-friendly bioplastics [20]. Following the other view, the easiest way for the classification of biodegradable polymers is based on their natural and synthetic origin. Natural biodegradable polymers are from polysaccharides, proteins, and microbes whereas synthetic biopolymers are the polymers developed by microbial fermentation, or through biotechnological production [75]. With regarding biopolymers’ response to thermal conditions, they can be divided into elastomers, thermosets, and thermoplastics [76]. The biopolymers are employed in specific fields depending on their cost, availability, moisture absorption, thermal stability, mechanical behaviour, degradation stability, and biocompatibility. PLA and PHAs are the two widely used biopolymers in production and application [77]. The major advantage of biodegradable polymers over nonbiodegradable polymers is their degradation by microorganisms which yield them to soil and enrich it. This stabilizes the environment and decreases the trash volume. The degradation capacity of biodegradable polymers is dependent on many factors including the type of polymer, chemical composition, and environmental conditions [78]. Despite wider applicability, a few shortcomings of biopolymers are their hydrophilic nature, low mechanical strength, and lower degradation rate in moist environments [79]. The following section details the classification of biodegradable polymers including both biopolymers and petroleum derived biodegradable polymers to present a wide variety of biodegradable polymer present.
3.1. Classification of Biodegradable Polymers
The classification of biodegradable polymers can be done on the bases of their origin, synthesis method, chemical composition, and applications. Broadly, they can be classified based on their origin as (1) natural polymers which are derived from renewable recourses like plants, animals, and microbes; (2) biodegradable polymers synthesized from petrochemicals products [80]. According to Siracusa et al. biodegradable polymers can be classified into three main categories: 1. Natural biodegradable polymers which are derived from natural and renewable resources like polysaccharides (starch, cellulose), lipids (oils), and proteins (silk, wool); 2. synthetic biodegradable polymers which include PLA, and polycaprolactone (PCL); 3. polymers obtained by microbes and genetically modified bacteria, for example, poly(hydroxyalkanoates) (PHAs) [81]. Figure 2 shows a classification of the biodegradable polymer. Since this report is mainly focused on bio-based polymers or biopolymers that are either obtained directly from bioresources or produced synthetically from the raw material obtained from bio-based resources. These are completely biodegradable and renewable. Some of the major biopolymers which are obtained from renewable bioresources are discussed. 3.1.1. Polylactic Acid (PLA)
PLA is one of the promising and widely used bio-polymers in the past few decades [82] due to its renewability, non-toxicity, abundant availability, low-cost, excellent mechanical properties [83], and incredible versatility of applications in the textile, chemical industries, food packaging, and pharmaceuticals [84]. PLA is a non-toxic linear aliphatic polyester synthesized using lactic acid (LA) as a monomer. Industrially LA is produced by chemical and microbial fermentation processes [85]and can be converted into PLA through condensation and ring-opening polymerization of cyclic lactide.
3.1.2. Poly-hydroxy-alkenoates (PHAs)
PHA is an environmentally friendly, biopolymer, produced from fatty acids, sugar, sucrose, molasses, starch, wheat, methane, and maize while the main source for commercial production is sucrose and glucose [86]. Natural PHAs are a category of polymers that have existed in nature for more than a million of years. Natural PHAs are formed by bacterial fermentation. PHAs have a wide range of applications due to their high performance, biocompatibility, and biodegradability [87]. According to a Mimicking Nature report at the end of 2021, a manufacturing organization is producing about 48 kilo tonnes of PHA annually. Mostly PHAs are used for biomedical applications such as tissue engineering and drug delivery [88].
3.1.3. Cellulose
Cellulose is the most abundant biopolymer found both in plants and animals while mainly extracted from plants. It can also be produced by a bacterial process [89]. Applications of cellulose vary greatly from composites, electronics, and energy materials to implants and tissue engineering [90]. It is the most utilized material in the world [91]. The annual production of cellulose, as per a 2018 report, was 180 million tonnes which is increasing rapidly annually [92].
3.1.4. Poly (lactic-co-glycolic Acid)
Poly (lactic-co-glycolic acid) (PLGA) is a synthetic renewable biodegradable copolymer of PLA and polyglycolic acid (PGA) synthesized via ring-opening copolymerization. PLGA possesses excellent biocompatibility, favourable biodegradation behaviour, and good mechanical strength. PLGA is an FDA-approved biopolymer and is extensively used for drug delivery, tissue engineering applications, bone scaffolding, and other biomedical applications [93].
3.1.5. Bio-epoxy Resins
Bio-based epoxies are bio-resins produced by epoxidation of renewable precursors such as unsaturated vegetable oils, cellulose, and biofuel productions like sorbitol derived from corn starch, glycerine, and glycerol from triglyceride vegetable oil [94]. Mechanical, and thermal properties of biobased epoxies are comparable to conventional non-biobased epoxies making them applicable to all conventional epoxy applications such as coatings, fiber-reinforced composites, and adhesives [95]. Some commercialized bio epoxy resins are liquid epoxidized natural rubber, epoxidized linseed oil, epoxidized cardanol, and Epicerol [96].
3.1.6. Poly (Butylene Succinate) (PBS)
PBS falls into the category of important commercial bio-derived polymer. Succinate-derived biopolymers include synthetic biodegradable polyesters like poly (ethylene succinate) (PES), and poly (butylene adipate) (PBA). They were generally not included in biopolymers as they were derived from petroleum and fossil bases resources but, in last decade,efforts have been made to derive them from bio-resources such as sugarcane, corn, and cassava. PBS is formed by a polycondensation reaction of succinic acid (SA) and 1,4-butanediol both of which can now be derived from bioresources making the PBS a biobased polymer [97]. Polymers detailed in this section are the most studied biopolymers for biocomposites and green composites fabrications. For preparation of biocomposites and green composites plant based natural fibers are majorly used. After a brief introduction about biocomposites and green composites, a detailed discussion about the raw materials which included natural fibers and biopolymers in various sections.
Figure 2. Classification of biodegradable polymers.
4. Green Composites/Bio-composites
There are several definitions for biocomposites and green composites given by several authors such as Md. Ibrahim [98] defined biocomposite as a composite material made up of matrix phase obtained from a sustainable and renewable source and natural fibers as reinforcement. Deepa et al. defined biocomposites as prostheses, biocomposites are either made up of bio-based material as the matrix and afiller scattered throughout it, or a structure of alternating portions of two or more types of biomaterials [99]. According to Abdulkhani et al. biocomposites are materials obtained from natural and renewable resources by combining two or more phases [100]. In general, most of the studies define biocomposites as natural fibers reinforced biopolymers. Natural fibers as the discontinuous phase are added to the biopolymer matrix in the continuous phase to improve the mechanical, physical, thermal, rheological, and acoustic properties of biopolymers [101]. The purpose of preparing biocomposites is to replace conventional non-biodegradable and non-sustainable composites with good mechanical properties and durability performance imparted by natural fiber and biopolymer, respectively. Usually, the maximum stiffness and tensile strength of biocomposites range between 1 to 4GPa and 20 to 200 MPa, respectively [77]. There are many studies where they obtained stiffness upto 8–10 GPa [102] and tensile strength higher than 200 MPa. Some of the studies obtained much better mechanical properties than conventional polymeric composites [103]. Soykeabkaew et al. prepared all cellulose composites which exhibit an average tensile strength of 910 MPa and Young‘s modulus of 23 GPa [104]. The chemical constituents, molecular weight, morphological characteristics, mechanical attributes, and processing technique of biocomposites are governed by the biopolymer part of the composite [76]. Mechanical and thermal properties obtained after processing, greatly depend upon the interaction between fibers and matrix [105] whereas in the case of biopolymeric blends as matrix, the properties also depend on the interaction between both the polymers [106]. A lot of work has been done on the development of biocomposites that could replace conventional non-biodegradable and non-sustainable polymeric composites in every sector. Already they are being applied in the construction, sports, packaging, healthcare, automotive, and aerospace industry [107]. Some recent studies done on biocomposites are given in Table 3.
Table 3. Recently reported biocomposites mentioning the polymer, filler and fabrication techniques used.
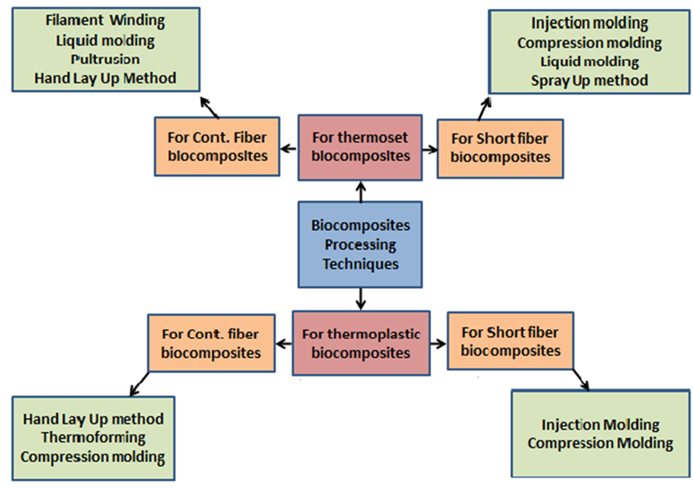
5. Processing Techniques for Preparation of Biocomposites
Several techniques are used to process natural fiber-based biocomposites which are similar to the method used for the fabrication of conventional polymer synthetic fiber composites. The selection of processing technique depends on many factors such as material, fiber type and dimension, the temperature of the composite, desired properties, surface parameters, and production cost [118]. Some of the major techniques used by the manufacturers and researchers are discussed in this section.
5.1. Hand Layup Method
This is the oldest, simplest, cheapest, and most common method to produce composites. First, an anti-adhesive agent is applied over the mould for easy removal of the composite, after which fibers are either chopped, powdered, or definite length placed in the mould. The biopolymer resin is then applied to the fibers using rollers, brush or simply pouring for preparing the first layer of composite. Other layers can be made over the first layer similarly [118]. Any entrapped air in between the layers is removed either by squeeze rolling or vacuum bag technique [119]. Drying or curing is then performed by leaving under standard atmospheric conditions. The hand layup technique is sometimes used with compression moulding and 3D printing as alternate layers of matrix material can be 3D printed and fibers then laid manually using hand. Prepared layers can be compressed to get better mechanical properties. The spray lay-up method is a modified version of hand lay-up, resin is sprayed on fibers instead of applying using a brush or rollers [120]. Gupta and Singh [121] produced sisal fiber reinforced PLA composites by hand lay-up technique. The tensile strength and tensile modulus of composite values increased from 30 MPa and 2 GPa to 35 MPa and 2.6 GPa. Sukmawan et al. [122] used bamboo fibers to prepare hand laid up composites with PLA matrix in cross ply orientation. First bamboo fibers were dipped in PLA solution and kept in sheet forms. These sheets were than dried, stacked in cross ply orientation and hot pressed at 165 °C. Tensile strength and modulus increased from 35 to 223 MPa and 2 to 10.5 GPa. Azlin and team [123] also used hand lay-up process to prepare PLA/polyester/kenaf fibers layered composites by hand laying PLA, polyester fiber sheet followed by heating in mould. This hybrid composite shows highest tensile strength of 103 MPa but a low tensile modulus of 0.6 GPa.
5.2. Solution Casting
This process involves the dissolution of biopolymer using a suitable solvent followed by adding filler to this polymer solution under stirring condition. After mixing properly, this mixture is then cast into desired shape mould and left undisturbed for slow drying either ambient or using an air oven. It is the most suitable for the fabrication of biocomposite films with short fiber or filler in powder form [124]. Proper mixing and slow drying are necessary to achieve high strength composites. The drying temperature should be lower than the boiling point of the solvent during mixing and drying otherwise bubble formation occurs which gets trapped in composites and leads to inferior mechanical properties [125]. Khan et al. prepared PBS/PLLA/HAP biocomposites film for bone scaffolding application using solvent casting. Both the polymers and HAP were dissolved in chloroform and then mixed under stirring condition. Tensile strength also increased from 43 MPa of PLLA to 51 MPa for composite [126]. Kale et al. [127] reinforced chitosan with silk fibers using solution casting process. Tensile strength and tensile modulus both increased with increase in fiber content. For 7 wt.% fiber, tensile strength and tensile modulus were 125 MPa and 2.1 GPa whereas for 12.5 wt.% fiber, these values increased to 200 MPa and 4 GPa. Liu and team [128] produced nanocellulose reinforced PLA green composites using solution casting process. Tensile strength and tensile modulus increased from 22 MPa and 1.5 GPa to 32 MPa and 2.2 GPa, respectively. In another study, Oyeoka [129] extracted nanocellulose crystals from water hyacinth fibers and used them as reinforcement for PVA/gelatine polymer blends to develop nanocomposites using solution casting by dissolving both PVA and gelatine into water. The tensile strength of PVA and gelatine blend increase from 7.91 to 11.73 MPa after the addition of cellulose nanocrystals into it.
5.3. Extrusion
One of the most widely used techniques to produce large products takes filler material either in chopped or powder form and polymer in the form of pellets. Many natural fiber-based bio-composite studies have been performed using single screw extrusion and twin-screw extrusion preferably because of throughout mixing of filler material in the matrix resulting in better mechanical properties [110,111]. This technique is also best utilized to produce polymer blends and to make hybrid composites as it mixes material intensely using heat [112]. This is also used in conjunction with other techniques such as injection moulding and additive manufacturing to properly mix different constituents of biocomposites before giving them a shape. Etxabide et al. [113] developed gelatine based porous biocomposites using double screw extrusion and injection moulding to show the use of such industrial techniques for the development of gelatine-incorporated lactose porous biocomposites. Yokesahachart and Yoksan [130] developed jute reinforced starch/PLA/PABT blend green composites using twin screw extrusion process. Tensile strength of composites is similar to pure polymer blend which is nearly 43 MPa, whereas tensile modulus value of composites increased with increase in jute content from 2.3 GPa at 0 wt.% jute to 3.5 GPa at 15 wt.% jute. Vandi and his team [131] used wood fiber with PHBV to prepare biocomposites using extrusion process. The tensile strength of PHBV decreased as content of wood fiber increase from 32 for pure PHBV to 20 MPa whereas value of tensile modulus increased from 3 GPa for pure PHBV to 4.3 GPa for 40% fiber content in PHBV. In another study [132], PABT was reinforced with hemp powder using twin screw extrusion. The tensile strength of PABT increased more than 4 folds from 6 MPa for neat PBAT to 25 MPa PBAT consisting 40% filler. The tensile modulus value for the same increased from 79 to 505 MPa.
5.4. Injection Moulding
Injection moulding is the most utilized approach for the manufacturing of plastic composites, especially for mass production. To produce natural fiber-based bio-composites, biopolymers can be used in the form of pellets or resin and natural fibers in chopped or powder form [133]. There are numerous studies on injection moulded biocomposites as they provide the final product directly from raw material without applying any solvent and post/pre-processing requirement [134]. Composites processed by injection moulding exhibit very good mechanical, and physical properties, and blending and shaping occurs inside a chamber with temperature and pressure [135]. Researchers have also used long fibers for injection moulding by implanting them in the mould before injecting a polymer into it. Okubo et al. [115] prepared bamboo/micro fibrillated cellulose PLA hybrid biocomposites for which they planted bamboo fibers in a mould before injecting cellulose-incorporated PLA resin into it and obtained better mechanical properties. Macaíba fiber reinforced PCL biocomposite was manufactured by Siqueira and team [111] through injection moulding process. The highest tensile strength was obtained 17.8 MPa and elastic modulus value of 191.85 MPa. In another study by Benjamin Bax [136], PLA was reinforced with Cordenka fibers using injection moulding process. Tensile strength and tensile modulus values obtained are 58 MPa and 4.8 GPa, respectively. Huda and co-workers [137] used extrusion followed by injection moulding to develop recycled cellulose added into PLA green composites. Mechanical properties of prepared composites were improved slightly from 62 MPa for pure PLA to 70 MPa for composite while Young’s modulus value increased more than two folds from 2.7 to 6.5 GPa.
5.5. Compression Moulding
Compression moulding uses compression with heat to melt press the matrix and filler into a simple desired shape and is very well suitable for laminates and flat composites. Simple machine includes a flat metal plate with a hydraulic press and heaters between which laminates can be placed and pressed at desired temperature and pressure. The parameters which need to be considered for this technique are pressure, temperature, time of heating, and amount of material. One can use direct pellets or resin with filler material while using plates with mould cavities that produce end material. Different layers of matrix and filler can be processed in flat plate configuration [138]. Bacterial-modified wheat straw-reinforced PVA biocomposites were prepared and characterized by Asgher and his team using the compression moulding method. The fibers were first delignified using bacteria to improve the crystallinity of fibers after which fibers and PVA were blended and moulded using a hot press in a preheated metallic mould at 60 °C for 20 minutes. The mechanical properties were determined using dynamic mechanical analysis (DMA) and achieved 64 MPa of modulus and a tensile strength of 2.7 GPa [139]. Ochi and his team [140] prepared Kenaf fiber reinforced PLA composites using compression moulding having tensile strength of 223 MPa and tensile modulus of 22 GPa. Porras and Maranon [141] developed bamboo fiber reinforced PLA composites through compression moulding process and obtained maximum tensile strength of 77 MPa. Eucalyptus fiber reinforced PBS composites were prepared using compression moulding by Nanthananon and team [142]. About 1.5 fold increase in tensile modulus value of PBS was obtained after the addition of eucalyptus fiber but the increase in tensile strength is only about 2 to 4%.
5.6. Resin Transfer Moulding
Resin transfer moulding involves transfer of low viscosity resin into rigid mould containing reinforcement using either higher pressure or vacuum. This resin infiltrates the reinforcement to convert into composite after curing. This process results in high quality surface finished products with excellent dimensional accuracy. Mostly used for thermoset polymer composites but recently some studies using resin transfer moulding (RTM) process for thermoplastic resins has been reported. This method includes various modifications such as resin transfer method, type of mould cavity, combinations with autoclave, and vacuum-assisted resin infusion [43]. Nils Cuinat and team [143] prepared flax fibers reinforced bio-based epoxy composites using high pressure assisted resin transfer moulding process. First, stacked flax fiber sheets in a closed mould were fabricated followed by pushing of resin to inside through inlet of mould. The mould was then heated for 6 h at 50 °C for cuing. The bending strength of composite appeared at around 24 MPa and the compressive strength for the composite was 135 MPa. Phuong Tran and his team [144] used RTM to produce kenaf fiber reinforced soyabean oil-based epoxy. The fibers were kept inside mould and covered with a vacuum bag film. The resin then sucked through inlet using a vacuum pump into the kenaf fibers stack. It was then cured at 150 °C for 3 h. The samples exhibit tensile strength of 7 MPa.
5.7. Filament Winding
Filament winding is an open mould process in which the fibers are first wetted in desired resin and then wrapped uniformly and regularly around a rotating mandrel. The fibers are supplied from a tow. The fiber pattern in composite can be changed by changing wrapping direction or angle. The resulting laminate is cured by pre-set process. Filament winding can be done in two ways either wet winding or dry winding. In wet winding, the fiber gets unwound from tow pass through resin before wrapping on mandrel. In dry winding fiber are pre-impregnated that is tow preg composite fabric. It is mainly used to produce hollow, circular, and prismatic structures such as pressure vessels, tanks, pipes, etc. Majority thermoset resins such as epoxy, phenolic, and polyester are used. However, there are studies that used thermoplastics resins for example PLA and natural fibers to prepare composites using filament winding. Reinhardt and team [145] created PLA-Viscose (continuous cellulose fibers). The tensile strength and modulus increased from 59 to 134 MPa and 3.6 GPa to 8.5 GPa, respectively. In another study, PLA was reinforced with hemp fibers. First prepregs of PLA/Hemp were prepared using wrap spinning method and then composites were prepared using compression moulding. The tensile strength and modulus increased from 41.2 MPa and 2.1 GPa to 72.75 MPa and 8.77 GPa, respectively [146]. Other than PLA researchers have also prepared bio-based epoxy with jute as reinforcement [147]. Four-point bending tests were carried out to obtain stress vs. strain curves of the samples. The highest flexure modulus obtained for the sample was 6 GPa whereas the highest bending strength was 70 MPa.
5.8. Additive Manufacturing/3D Printing
3D printing is one of the most recent and advanced fabrication technologies for almost all kinds of materials applications from metals to soft tissues [148]. It includes layer-by-layer addition of material to create an object with the aid of a computer-based 3D model [149]. Recently, 3D printing for the synthesis of polymer-based composites has drawn a lot of attention from researchers therefore numerous studies have already been conducted using this technique [150]. The most used techniques in 3D printing are fused deposition modelling, Stereo Lithography Apparatus, and selective laser sintering. PLA-based biocomposites are the most preferred biopolymer for 3D printed biocomposites as PLA is the easiest biopolymer to print due to its low melting point and minimal wrapping during printing [151,152]. Niang et al. developed typha stem powder incorporated PBS biocomposites using the fused deposition modelling technique and obtained 220% and 134% increments in tensile strength and tensile modulus, respectively [153]. Matsuzaki and coworkers [154] developed jute fiber reinforced PLA composites using additive manufacturing specifically fused-deposition modelling technique. The mechanical properties such as tensile strength and modulus were improved from 48 MPa to 53 MPa and 3.5 GPa to 5.2 GPa. In another study, [155] bamboo and flax fiber reinforced PLA composites was prepared using additive manufacturing. The fibers were first cut into short fibers and then fed to extruder along with PLA. Composites with different weight fractions of flax and bamboo were developed. Tensile properties reduced to 2–3 folds as compared to neat PLA that is from 65 MPa for neat PLA to 28 for bamboo reinforced PLA and to 30 MPa for flax reinforced PLA. However, tensile modulus for bamboo reinforced fibers improved up to 29 to 39%. Agaliotis and team [156] used henequen fibers to reinforce PLA using additive manufacturing. A decrease in tensile strength from 56 MPa for pure PLA to 48.6 MPa was observed in composite. Tensile modulus values also reduced from 1.93 to 1.62 GPa. The decrease in tensile properties is possibly due to voids creation during deposition. Classification of processing techniques are presented in Figure 3 and Figure 4 based on the type of mould and fiber dimension.
Figure 3. Classification of processing techniques on the bases of types of mould.
Figure 4. Classification of processing techniques on the bases of fiber length.
6. Characterization of Biocomposites
After fabrication, the most important step is to test and characterize the composites to check whether it is suitable for real-life applications with the desired properties. Characterization and tests are also important for comparing the product with already existing products, to remove the faults of fabrication by identifying it through various tests to produce better material [157,158]. This is an important process to probe composite microstructure, physical, chemical, and thermal properties using various analytical methods. For natural fiber-reinforced bio-composites, various tests and characterizations usually performed to probe the material which are listed below.
6.1. X-ray Diffraction
X-ray diffraction (XRD) is used to determine the structure, phase identification, and crystallinity of the composites to understand the effect of fillers [159]. Researchers used XRD for the crystallinity study of natural fibers [160]and for change in crystallinity in composites after reinforcing polymer with natural fiber [161]. Abraham et al. [162] characterized nanocellulose reinforced natural rubber composite using XRD and observed an increase in crystallinity with the increasing nanocellulose content in rubber which was previously showing a completely amorphous nature. Figure 5a shows XRD patterns of Napier grass stem and the products formed after alkali, bleaching, and KMnO4-oxalic acid redox reaction treatment done on Napier grass stems to obtain carboxylated nanocellulose crystals [163]. The characteristic peaks shown at 15.5°, 16.3°, 22.2°, and 34.6° in the figure correspond to cellulose crystallographic planes with miller indices values of (110), (110), (200) and (004) [164,165]. After each step of chemical treatment, the crystallinity of fibers increased to 73.3% as compared to 57.5% for raw grass fibers, after the final redox treatment which is supported by other studies as well [166].6.4. Mechanical Responses
Mechanical tests ensure whether the material fulfils the performance requirement according to industrial specifications, especially for the aerospace, automotive, medical, and defence industries [179]. These tests include tensile, flexural, impact strength, compression, and bending tests of the composite carried out according to various standards such as ASTM D638 for the tensile test [180], ASTM D790 for flexural properties [181], ASTM D256 for the impact test [182]. Tensile, flexural, compression, and bending are done using a universal testing machine whereas the impact strength test is determined by Charpy and Izod impact test [183].
6.4.1. Tensile Test
Tensile test is a basic test comes under mechanical tests to determine how a material behave when tensile forces are applied on it. It is used to measure how much of tensile load one material can sustain before permanent deformation and before fracture. Properties that can be measure directly by tensile test are yield strength, ultimate tensile strength and elongation at break. All sorts of materials designed for engineering applications need to sustain some amounts of tensile load. For each class of materials there are some pre-defined standards describing test specimen dimensions and test parameters [184]. For composites some specific standards are ASTM D 638, ASTM D 3039, ISO 527-5 [179]. Tensile tests are performed using a universal testing machine (UTM) and it is being used to determine tensile strength, elastic modulus and elongation at break properties of the samples. Most of the studies on natural fibers based green composites or biocomposites include tensile test. Ismail and team [185] prepared bamboo fibber reinforced natural rubber composites with different weight content of bamboo fibers. To evaluate the tensile properties, they performed a tensile test using UTM as per ASTM D412 standard. Both tensile strength and elongation at break decreased from 19.8 MPa and 749% to 3.6 MPa and 181% at 50 wt.% of bamboo fibers in natural rubber. Whereas tensile modulus value increased from 0.61 MPa of neat rubber to 1.91 MPa at 50 wt.% rubber. Similarly, PLA was reinforced with nanocellulose crystals [186]. It was found that with increase in nanocellulose content in PLA both tensile strength and tensile modulus increased up to 10 wt.% of nanocellulose. The tensile strength and modulus for pure PLA was 20 MPa and 2 GPa which increased to 37 MPa and 3.6 GPa, respectively. Figure 7a displays the stress-strain curve of low, medium, and high molecular weight film of neat chitosan, and Figure 7b shows a stress-strain curve of chitosan-flax fiber laminates prepared by Rath et al. [112]. It can be seen that tensile strength of high molecular weight chitosan film is higher than that lower and medium molecular weight polymer whereas the low molecular weight chitosan flax laminates have higher tensile strength than the others and overall neat chitosan films have better tensile strength than its laminates with flax fiber mats.6.4.2. Bending Test
Bending tests are performed to determine bending modulus, flexure strength and flexure strain of materials. They are also used to determine the tensile strength of brittle materials which are difficult to test using tensile test. Most commonly used bending tests are three point bending test, four point bending test, cantilever test, and G-torsion test [187]. Bending tests are very common experimental characterization in industry as engineering materials face bending stresses more commonly throughout their service life. For composites especially laminates and sandwich, composites bending tests are of much importance to determine interlaminar strength. During bending test, material can be tested both in cyclic loading and quasistatic loading [188]. Three points bending and four-point bending tests can be performed using UTM. ASTM D790 and ISO 178 are some standards for three-point bending tests. Most researchers include these test to evaluate the flexural strength of prepared composite [189]. Habibi and his team [190] performed a flexure test on different orientations laminates prepared using flax fiber reinforced biobased CORAL resin prepregs prepared through hand layup technique. Flexure test was done to understand how stacking sequence affects the laminate bending behavior. Stacks placed in unidirectional sequence were found to have highest flexure modulus and strength of 26 GPa and 244 MPa. Figure 7c shows flexural strength vs. strain of chitosan-flax fiber laminates. Laminates made up of medium molecular-weight chitosan have higher flexural strength than higher and lower molecular-weight chitosan laminates [112].
6.5. Morphology
Morphology involves microscopic imaging of biocomposites at various resolutions to see and understand features such as shape and size of fillers, distributions of filler, pores, and cavities, the effect of various treatments on matrix and fillers textures, adhesion between filler and matrix using various imaging techniques like Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM), and Atomic Force Microscopy (AFM). Morphological analyses are also used to analyse the fracture profile of biocomposites and to better understand the influence of filler shape, and size distribution on composite’s properties.
6.5.1. Scanning Electron Microscopy (SEM)
SEM has been used for a wide range of polymer and composite studies and applications, including fiber matrix adherence, fiber distribution, fracture surface, phase boundary, surface roughness, and adhesive failure [191]. SEM provides high magnification and depth of field, enabling examiners to see fiber ends, surfaces, or transverse areas in detail [192]. Figure 8 shows the SEM image of Nappier grass fibers before and after treatment for the reinforcement in natural rubber to prepare biocomposites by Lorwanishpaisarn et al. [163]. Image ‘a’ shows the photographic image of ground grass fibers and image ‘e’ shows its corresponding SEM image. The presence of pectin and hemicellulose holds the fibers together forming such a large structure whereas partial elimination of pectin and hemicellulose took place after the alkali treatment and the fiber become brighter and smaller in size as shown by images ‘b’ and ‘f’. Bleaching of fibers was done to removed cementitious material such as hemicellulose, lignin, and pectin, and a further reduction in the size of fibers took place as shown by images ‘c’ and ‘g’ of Figure 8(i). Alkali and bleaching treatment leads to the deliberation of parts of fibers by breaking alkali liable linkages especially esters and ethers linkages which is supported by other studies as well [193,194]. After reaction with KMnO4 and oxalic acid, a white colour suspension was obtained shown in image ‘d’ of Figure 8. The suspension was then freeze-dried to obtain the final product in powder form consisting of micro and nanocellulose fibers shown in image ‘h’.
6.5.2. Transmission Electron Microscopy (TEM)
Transmission Electron Microscopy (TEM) offers a unique combination of analytical techniques such as electron energy loss spectroscopy (EELS), energy dispersive X-ray spectroscopy, bright and dark field imaging for structural, and chemical analyses at the nanoscale level of the composites. Nam et al. [195] imaged the dispersion of silver nanoparticles in cotton microfibers using TEM. The image of the left edge, centre, and right edge of the cross-section of cotton fiber are shown in Figure 8(ii) [195].6.5.3. Atomic Force Microscopy (AFM)
AFM is one of the microscopic technologies with the greatest lateral resolution [196] used to analyse the surface roughness of biocomposites where the surface smoothness is being modified by various processes. Raj et al. used AFM for interfacial studies of PLA/flax fiber biocomposites to map adhesion properties of raw and NaOH treated fiber [197]. AFM can also be used to determine the mechanical properties of sub-micron spatial resolution [198]. Figure 8(iii)shows the AFM image of cellulose nanofibers synthesized via acid hydrolysis of bamboo fibers [199].
6.6. Rheological Studies
Rheology is the study of the flow behaviour of liquids and the deformation of solids under shear forces. The factors which influence the flow behaviour includes shear rate, shear stress, degree and duration of force applied and temperature. Rheology is an important tool to study the flow behaviour of viscoelastic fluids during the manufacturing process, and how the resin behaves during the impregnation of fibers during the manufacturing of fiber-reinforced composites [200]. Rheological properties include gel point, initial viscosity, and minimum viscosity. Initial viscosity is a helpful indicator of resin flow for quality control, it also indicates the onset of polymer melting and does not depend on the heating rate. Minimum viscosity gives an idea about the temperature range and period for which the resistance to flow is minimum. The rheological experiment also provides optimum heating rate, shear flow, and viscosity at different temperatures, pressure, and frequencies [201]. Figure 9 shows the flow curve with respect to angular frequency for Mater-Bi® and hazelnut shell powder biocomposites using a parallel plate rheometer. The viscosity of the polymer increased to 2 and 4 folds after the addition of hazelnut shell powder as filler and shows non-Newtonian behaviour which enhanced with increasing filler [116].6.7.3. Fungal Degradation
Fungi grow on the surface of the polymer by consuming it as food through oxidation or hydrolysis by enzymes released by fungi. These enzymes break down the high-molecular-weight polymer into low-molecular-weight monomers which could easily be transported through the cell wall of fungi. Environmental factors such as pH, temperature, and humidity affect degradation rate as with other biodegradation processes. These tests are majorly done in sealed containers. First fungi are grown, then samples to be biodegraded are inoculated with small pieces of these grown fungi inside a flask sealed with stoppers and degradation is observed by taking visual observation and by spectroscopic analysis of the samples [218]. Stoleru et al. performed the biodegradation of PLA/chitosan biocomposites by Phanerochaete chrysosporium fungus [219]. The changes in samples were characterized using FTIR, AFM, and gel permeation chromatography to determine the degradation that occurred after the fungi embedment.
6.2. FTIR Spectroscopy
Fourier transforms infrared (FTIR) spectroscopy is used to determine the surface chemistry, type of bonds, chemical composition, and functional groups present on the fiber and polymer. FTIR spectroscopy gives a characteristic pattern for organic materials and therefore quantitative and qualitative analysis can be done. Changes in surface chemistry after chemical treatment of fibers and chemical interactions between fiber and biopolymer are determined using FTIR spectroscopy [167,168]. Figure 5b shows FTIR spectra taken from a study for the extraction of nanocellulose fibers chemically from Napier grass stems [163]. Napier grass stem curve shows the presence of aromatic, esters, ketones, and alcohol functional groups in its spectra which is due to the presence of cellulose, hemicellulose, pectin, and lignin. O–H and C–H stretching frequencies in lignin and cellulose are shown by peaks at 3337 and 2920 cm−1. Peaks at 1735 cm−1 represents C=O in lignin and hemicellulose, and peaks at 1589 cm−1 represents C=C stretching in lignin [169]. After the alkali treatment of grass, the intensity of peaks in 1735 and 1240 cm−1 reduced significantly which shows a reduction in hemicellulose lignin contents. The peaks at 1240 and 1510 cm−1 almost disappeared, as further reduction in lignin content occurs after bleaching. There is not much change observed at the 1715 cm−1 peak after the redox reaction of bleached samples, which indicates the presence of carboxyl groups [163].
6.3. Thermal Characterizations
Thermal analyses of biocomposites and green composites are done to determine changes in physical and in some cases mechanical properties like change in weight, change in crystallinity, heat absorbed and released, and phase transition with varying temperatures. The major characterization techniques to evaluate the thermal properties of biocomposites are differential scanning calorimetry (DSC), thermogravimetric Analysis (TGA), and dynamic mechanical analysis (DMA). All these three thermal analyses techniques are discussed in the following sub-sections.
6.3.1. Thermogravimetric Analysis (TGA)
TGA is used to obtain information about the thermal stability and content analysis of polymeric materials in a temperature range of 25 to 600 °C [170]. A sample of known weight is kept inside the TGA’s analysis chamber where temperature rises gradually and the weight change is recorded against the temperature change. Satsum et al. [171] analysed PLA–pectin composites for which the TGA curves are shown in Figure 6a. PLA and its composites with 2% and 4% pectin show the single-step weight loss. Therefore, no initial weight loss was observed from moisture phenomenon that could take place, whereas in the case of PLA composites with 6% and 8% pectin, the weight loss happened in three main stages. In the first stage, elimination of absorbed moisture took place, in the second stage degradation of pectin in composite took place was 50% weight loss happened and finally in third stage depolymerization took place and major chain structure was broken with the evaporation of CO2, CO, and CH4 results in further 30% weight loss [172].
6.3.2. Differential Scanning Calorimetry (DSC)
DSC analysis is used to determine glass transition temperature, crystallization temperature, melting temperature, heat capacity, and enthalpy variation of polymer materials and their composites at varying temperatures during the heating and cooling cycles and phase transitions. For the case of natural fiber biocomposites, several studies stated no change in glass transition temperature but a shift in melting point (Tm), crystallization temperature (Tc), and percentage crystallinity before and after incorporation of natural fibers in biopolymers [173,174]. DSC curve of PLA, diatomaceous earth (a naturally occurring sand made up of fossilized remains of small aquatic organisms called diatoms), and bee wax biocomposites are shown in Figure 6b [175]. Neat PLA is not showing any glass transition temperature or crystallization temperature but only a small endothermic transition at 100 °C whereas its composite with diatoms (silicified algae) shows a glass transition temperature at 60 °C along with a crystallization peak. PLA containing diatomaceous earth (dark green) as filler is not showing any crystallization peak but the crystallization peak is observed after the addition of bee wax in PLA. The enthalpy of crystallization and melting temperature increased with the addition of bee wax representing an increase in the crystallinity of PLA [175].
6.3.3. Dynamic Mechanical Analysis (DMA)
DMA is used to determine the viscoelastic behaviour of advanced materials by determining storage modulus (G′), loss modulus (G″), and damping factor (tanδ. It is mainly used for polymeric materials to better understand the glass transition temperature (Tg) [176]. The G′ values determine the stiffness of the material, and loss modulus (G″) represents the energy loss in the form of heat due to intermolecular friction. The damping factor (tanδ) is the ratio of loss modulus to storage modulus and determines the damping behaviour of the material as a function of temperature. Glass transition temperature can also be determined from the damping factor profile [177]. These can be used to determine the influence of various treatments given to fibers on the thermal properties of biocomposites [178]. Figure 6c,d shows DMA results of mater-Bi® and hazelnut shell powder biocomposites studied by Ceraulo et. al. [115]. The storage modulus value of biocomposites increased with an increase in the content of filler. Similarly, tanδ curves show a sudden increase in loss factor at glass transition temperature which is a typical behaviour of polymeric materials. It can be seen that neat polymer shows a higher damping factor value, while after the addition of filler, it reduced many folds and the glass transition temperature also shifted towards higher values due to the presence of filler.
Figure 6. (a) TGA profiles of PLA blend with different compositions of Pectin taken with permission from Ref. [137]; (b) DSC curves of PLA and its composites with 10% diatoms reproduced with permission from Ref. [141]; (c) and (d) storage modulus and damping factor of mater-Bi® and hazelnut shell powder biocomposites reproduced with permission from Ref. [102].
Figure 7. (a) stress-strain curves of pure chitosan films; (b) stress-strain curves of chitosan-flax fiber laminates; (c) flexure stress-strain curves for chitosan-flax laminates; and (d) shows the comparison of specific modulus vs. specific strength of prepared laminates with other composites and polymers. Taken from Ref. [99] with permission.
Figure 8. (i) SEM and photographic images of Napier grass before and after each chemical treatment, reproduced with permission from Ref. [130]; (ii) TEM images of cross-section of silver- cotton nanocomposites reproduced with permission from Ref. [155]; and (iii) AFM image of Nano Cellulose fibers reproduced with permission from Ref. [159].
6.7. Biodegradation Studies
Biodegradation is a biochemical process that involves in breakdown of organic materials into a simpler form compound, mineralized, and redistributed into the environment by elemental cycles such as the carbon, nitrogen, and sulfur cycles. It only occurs within a biosphere and involves hydrolytic cleavage of chemical bonds by microorganisms under certain environmental conditions [202]. It differentiates from other degradations such as thermal, photo, and weathering because all of these occur under harsh conditions and without microorganisms [203]. Ali et al. [204] showed the gradual breakdown of material by specific biological activity. Biodegradation occurs through bio-fragmentation, bio-deterioration, and assimilation [205]. The factors that affect the biodegradability of polymers and their composite are divided into two groups; one is their physical properties which includes molecular structure, molecular mass, and crystallinity of the materials; and the second group is the environmental conditions that include temperature, humidity, pH and the types of microorganisms. The biodegradation test of biocomposites is done to evaluate the behaviour of composite material in different conditions, the time it takes to degrade, and how physical and mechanical properties deteriorate with degradation [206]. Biodegradation of biopolymers or biodegradable polymers can also be controlled by adding various fillers such as nanoclay [207,208]. Controlled biodegradation is particularly used for drug delivery [209]. The most common test methods for biodegradation studies are enzymatic degradation and soil burial tests. Microbial tests such as fungal tests are also used by some researchers to test the biodegradability of biocomposites as fungal attacks are more common. Detailed explanations about enzymatic, soil burial, and fungal degradations tests are given below.
6.7.1. Enzymatic Degradation
Degradation of polymeric material occurs through the breakdown of chemical bonds by the action of certain enzymes. The enzymatic degradation mainly happens in two steps:adsorption of enzymes on the surface of the polymer and then, hydro-peroxidation or hydrolysis of the bonds [210]. The enzymatic degradation of PLA occurs through hydrolysis of the ester bond backbone for which the most studied enzymes are Protinease K, Lipase, and Esterase. Its degradation greatly depends on pH and temperature [211]. A study done by Xu and co-workers demonstrated that PLA in physiological pH of 7.4 took 100 h to degrade, but when tested at acidic pH of ~3, the degradation occurred in 400 h. Similarly, at 37 °C the degradation rate was 4 times faster than the degradation rate at 25°C [212]. Stepczyńska et al. performed an enzymatic degradation test over flax fibers reinforced PLA composites using four different enzymes namely Proteinase K, Lipase, Protease, and Esterase. Results showed that Proteinase K is more effective for the degradation of PLA and its composites than other enzymes. Other studies also found that the activity of Proteinase K is much higher than other enzymes for PLA and its composites [213]. Figure 10(i) shows SEM images of enzymatically degraded PLA/PBS blend films performed by Shi et al. [214] using an enzyme Cutinase. There are no pores initially, some pores are seen after 4 days of degradation. As time passes these pores are getting denser and bigger throughout the material, also with an increase in PBS content pore sizes are getting bigger. For PBS/PLA20/80, PBS/PLA30/70, PBS/PLA40/60, and PBS/PLA50/50 the mean pore sizes are 4.09, 7.58, 8.51, and 110 µm on the 8th day of selective Cutinase hydrolysis.
6.7.2. Soil Burial Test
To carry out soil burial biodegradation of the composite, samples are buried under soil consisting of manure and sand in specific ratio kept in ambient conditions or controlled conditions. The weight of samples was measured before the test and samples are taken out at particular time intervals to measure their weight again. Degradation occurs by the action of microbes present in the soil. Sample filled with natural fibers shows faster degradation than neat samples as natural fiber makes the matrix porous to ease for microbial attack. Like other biological processes, soil burial test results are affected by factors such as temperature, humidity, pH, light, and airflow [215]. Yaacob et al. performed soil burial test on PLA/ rice straw powder biocomposites in natural soil under ambient conditions of humidity and temperature and achieved 50% weight loss in 6 months for the samples consisting of maximum filler (20wt.%) [216]. The slow degradation of PLA is also proved by another study done by Slezak et al. [217]. PLA and its composite with PBS were buried under natural soil under ambient conditions. No degradation could be seen in 12 months, shown by SEM images as in Figure 10(ii). No surface erosion or pores were observed after 6 months of burial and some changes on the surface could be seen only after 12 months of soil burial.
Figure 10. (i) SEM images of PLA/PBS blends at different PBS content in PLA before and after enzymatic degradation, reproduced with permission from Ref. [174]; (ii) SEM images of PLA and PBS before and after soil burial; PLA_1 is a composite of PLA with polybutylene adipate terephthalate and additive, PLA_2 is a composite of PLA and minerals fillers, and PBS_1 is a composite of PBS and mineral additives. Reproduced with permission from Ref. [177].
7. Applications of Biocomposites
High specific strengths, low cost, easy processing, abundance of raw material, and environmental friendliness make biocomposites an excellent choice as the replacement for petroleum-based polymeric composites for wider applications. Their applicability has been improving for the last three decades which is now catching high pace due to restrictions on non-renewable materials. The key sectors where biocomposites are being utilized most are food packaging, automotive, biomedical, construction, interior, and sports. Their high specific strength and lightweights are best suitable for the automotive and aviation industry [220]. Some of the major car manufacturers like Toyota, Mercedez Benz, and Volkswagen have been utilizing natural fiber composite for the last 20–30 years now. Use of biocomposites and natural fibers to reduce vehicle weight by 10% and enhance fuel efficiency [86], reduce vibrations and noise [221]. Currently, biocomposites are used only for interior parts only where much higher loads are not required [222]. Around 8 million vehicles of Ford have seats made up of soy foam reducing fossil fuel usage by 5 million pounds per year [223]. Biocompatibility, optimum degradation kinetics, and flexible mechanical properties of biocomposites make them the best choice for biomedicine and medical-related applications such as implants, tissue engineering, bone scaffolding, wound healing, and drug delivery [173,174]. Natural fiber-based biocomposites such as wheat/rice straw mixed clay bricks have been used for construction for ages now. Superior thermal insulation, noise insulation, and vibration damping of natural fibers make their use in biocomposites an excellent choice for construction applications such as doors, window panels, heat shields for sunlight exposure, tiles, and roofing. They are being used in place of fiber glass-reinforced composites. Researchers have used hemp fiber with concrete to improve thermal and sound insulation [224] and other properties [225]. Packaging is one of the rapidly growing sector where the replacement of single-use nonbiodegradable plastics is needed [226]. Some major industries such as West Rock Company, Clearwater Paper Corporation, Stora Enso, and Bemis Manufacturing have started manufacturing biodegradable and green packaging for various applications. They are using different types of biopolymer blends such as starch, PLA, Pectin, cellulose, and natural fiber-based biocomposites for cosmetics packaging applications [227]. Some examples of biocomposites applied in various sectors are listed in Table 4.
Table 4. Biocomposites applied in various fields.
8. Current Challenges for Biocomposites
At present, several challenges are being faced by biocomposites which may have inconsistency in properties of natural fibers. Properties of natural fibers vary from place to place, from season to season, and even from plant to plant due to variations in environmental conditions, minerals in soils from place to place.Therefore, fabricating biocomposites with similar properties is very difficult which limit their industrial manufacturing and mass applications [240]. The high moisture sensitivity of natural fibers and biopolymers gives a big challenge in their use of packaging, and long time storage cannot be done as they are prone to microbial attack. Challenges during the fabrication arise due to moisture absorbing tendency which causes swelling, and shrinking during fabrication leading to improper distribution and poor adhesion with matrix in fabricated composites [241]. The lower thermal stability of natural fibers and biopolymers limits the high-temperature use in fabrication because most of the natural fibers degrade with a rise in processing temperature [242]. Fiber breakage during processing and entangled fibrils lead to poor distribution of fibers in biocomposites. Because of their lower thermal resistance, lower impact, tensile, and flexure strength, they are used only for lower load applications, where no higher temperatures or temperature fluctuations are needed [243]. These factors limit their high temperature and high load-bearing applications.
9. Future Scope of Biocomposites
The sole cause behind the lower mechanical properties of biocomposites is interfacial bonding between biopolymers and most natural fibers. Therefore, further research on the basic understanding of single fiber morphology, surface modification, and their mechanical behaviour is necessary to enhance interfacial bonding and fiber dispersion. Hundreds of studies exist on fibers surface modification with various treatments but none for single fiber processing which is very required to enhance their mechanical properties. More work is needed on new surface modification techniques, the use of coupling agents, and compounding techniques for biocomposite manufacturing. There is plenty of room for research on new and less common fibers which can enhance their applications, giving direct benefit to those rural societies where these materials are in ample quantity. Hybridization of biocomposites holds huge scope as only a few studies are available. Utilizing multiple types of natural fibers and polymeric blends in a synergetic approach is necessary to achieve more flexibility in fabrication and tailoring properties. This can expand the applicability of biocomposites in various fields. Few studies are present on polymer-metallic foam composites [244]. Biocomposites made up of biopolymers and renewable biodegradable synthetic materials such as metallic foams can be used to fabricate new-age biocomposites with enhanced mechanical and thermal properties. The inconsistency in the properties of biocomposites can be overcome by advanced manufacturing techniques such as additive manufacturing but a lot of improvements are required to make this technique suitable for the fabrication of a wide variety of biocomposites and continuous productions. Design and performance aspects that include product standards, lab-scale concepts, durability, and degradation models need to be focused on to increase the applicability of biocomposites. The commercialization of biocomposites is increasing and expected to grow more due to rapid environmental concerns and awareness among people about sustainability, advancement inprocessing techniques, and emerging new applications for biocomposites. Research on biocomposites needs to be enhanced globally. To enhance their applicability, biocomposites with high performance, durability, serviceability, and reliability need to be fabricated.
10. Conclusions
This review article gives an overview of biomaterials to prepare biocomposites, characterization methods, major processing techniques, recent applications, current challenges, and future scope of biocomposites. Detailed explanation of rheological, processing and biodegradation studies have been included in this review article on biocomposites. Increasing industrial interest in biopolymer-based biocomposites in various sectors needs greater attention towards their properties which not only include basic properties such as for conventional polymer composites but also their biodegradability and processing behaviour. Examples of recent studies are included showing the utilization of renewable fillers other than natural fibers are being used to enhance the applicability of biocomposites. In the last two decades, numerous developments have happened in the field of biocomposites and green composites, with their applications having increased from the biomedical field to automotive, aviation, construction, packaging, electronics, and sports. Researchers are trying to develop biocomposites with improved properties from the current state to extend their applicability further from being used in the interior only to the exterior and lower load applications to moderate load-taking applications. Numerous variations are used such as blending different types of biopolymers, hybrid biocomposites, various types of modifications, and advanced processing techniques paving the way for biocomposites to replace non-sustainable polymer composites.
Acknowledgement
The authors H.R., S.T. and S.S.M. acknowledge the institute for providing teaching assistantships. The author S.B. gratefully acknowledges University Grant Commission, India, for providing fellowship. The author A.M.C. gratefully acknowledges Council for Scientific and Industrial Research, India, for providing fellowship.
Author Contributions
H.R.: Investigation, conceptualization, writing original draft, S.T.: Investigation, conceptualization, formatting manuscript, S.B.: formatting, writing A.M.C.: Investigation, S.S.M.: Investigation, P.M.: Conceptualization, funding, editing.
Funding
This research was funded by Tata Innovation Fellowship (sanction no. BT/HRD/35/01/02/2020).
Declaration of Competing Interest
The authors declared no conflict of interest.
List of Abbreviations
PLA $$\,\,$$Poly (lactic acid)
PHA $$\,\,$$Polyhydroxyalkanoates
DNFI $$\,\,$$Discover Natural Fibers Initiative
OH $$\,\,$$Hydroxyl group
NaOH $$\,\,$$Sodium Hydroxide
PCL $$\,\,$$Polycaprolactone
PBS $$\,\,$$Poly (butylene succinate)
PES $$\,\,$$Poly (ethylene succinate)
PBA $$\,\,$$Poly (butylene adipate)
PLLA $$\,\,$$Poly (L-lactic acid)
HAP $$\,\,$$Hydroxyapatite
DMA $$\,\,$$Dynamic Mechanical Analysis
FTIR $$\,\,$$Fourier Transform Infrared Spectroscopy
DSC $$\,\,$$Differential Scanning Calorimetry
TGA $$\,\,$$Thermogravimetric analysis
G′ $$\,\,$$Storage Modulus
G″ $$\,\,$$Loss Modulus
ASTM $$\,\,$$American Society for Testing and Materials
SEM $$\,\,$$Scanning Electron Microscope
TEM $$\,\,$$Transmission Electron Microscopy
AFM $$\,\,$$Atomic Force Microscopy
PP $$\,\,$$Polypropylene
PET $$\,\,$$Polyethylene terephthalate
PHV $$\,\,$$Poly (hydroxy valerate)
PHB $$\,\,$$Poly (hydroxyl butyrate)
References
1.
Naghdi R. Advanced Natural Fibre-Based Fully Biodegradable and Renewable Composites and Nanocomposites: A Comprehensive Review. Int. Wood Prod. J. 2021, 12, 178–193. [Google Scholar]
2.
Suran M. A Planet Too Rich in Fibre. EMBO Rep. 2018, 19, e46701. [Google Scholar]
3.
Walker TR, Fequet L. Current Trends of Unsustainable Plastic Production and Micro(Nano)Plastic Pollution. TrAC Trends Anal. Chem. 2023, 160, 116984. [Google Scholar]
4.
La Mantia FP, Morreale M. Green Composites: A Brief Review. Compos. Part A Appl. Sci. Manuf. 2011, 42, 579–588. [Google Scholar]
5.
Nanda S, Patra BR, Patel R, Bakos J, Dalai AK. Innovations in Applications and Prospects of Bioplastics and Biopolymers: A Review. Environ. Chem. Lett. 2022, 20, 379–395. [Google Scholar]
6.
Baillie C. Why Green Composites? In Green Composites: Polymer Composites and the Environment; CRC Press: Boca Raton, FL, USA, 2004.
7.
Thakur VK, Thakur MK, Raghavan P, Kessler MR. Progress in Green Polymer Composites from Lignin for Multifunctional Applications: A Review. ACS Sustain. Chem. Eng. 2014, 2, 1072–1092. [Google Scholar]
8.
Mohanty AK, Misra M, Drzal LT. Natural Fibers, Biopolymers, and Biocomposites; CRC Press: Boca Raton, FL, USA, 2005.
9.
Kulhan T, Kamboj A, Gupta NK, Somani N. Fabrication Methods of Glass Fibre Composites—A Review. Funct. Compos. Struct. 2022, 4, 22001. [Google Scholar]
10.
Satyanarayana KG, Arizaga GGC, Wypych F. Biodegradable Composites Based on Lignocellulosic Fibers-An Overview. Prog. Polym. Sci. 2009, 34, 982–1021. [Google Scholar]
11.
Daculsi G. History of Development and Use of the Bioceramics and Biocomposites. In Handbook of Bioceramics and Biocomposites; Springer: Cham, Switzerland, 2016; pp. 1–20.
12.
Partanen A, Carus M. Biocomposites, Find the Real Alternative to Plastic – An Examination of Biocomposites in the Market. Reinf. Plast. 2021, 63, 317–321. [Google Scholar]
13.
Gurunathan T, Mohanty S, Nayak SK. A Review of the Recent Developments in Biocomposites Based on Natural Fibres and Their Application Perspectives. Compos. Part A Appl. Sci. Manuf. 2015, 77, 1–25. [Google Scholar]
14.
Karimah A, Ridho MR, Munawar SS, Adi DS, Ismadi; Damayanti R, et al. A Review on Natural Fibers for Development of Eco-Friendly Bio-Composite: Characteristics, and Utilizations. J. Mater. Res. Technol. 2021, 13, 2442–2458. [Google Scholar]
15.
Thakur VK, Singha AS, Thakur MK. Biopolymers Based Green Composites: Mechanical, Thermal and Physico-Chemical Characterization. J. Polym. Environ. 2012, 20, 412–421. [Google Scholar]
16.
Huang Z-M. Biocomposites. In Comprehensive Structural Integrity, 2nd ed.; Elsevier: Oxford, UK, 2023.
17.
Müssig J. Industrial Applications of Natural Fibres: Structure, Properties and Technical Applications; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2010.
18.
Faruk O, Bledzki AK, Fink HP, Sain M. Biocomposites Reinforced with Natural Fibers: 2000–2010. Prog. Polym. Sci. 2012, 37, 1552–1596. [Google Scholar]
19.
Sarikaya E, Çallioğlu H, Demirel H. Production of Epoxy Composites Reinforced by Different Natural Fibers and Their Mechanical Properties. Compos. Part B Eng. 2019, 167, 461–466. [Google Scholar]
20.
Noor Azammi AM, Ilyas RA, Sapuan SM, Ibrahim R, Atikah MSN, Asrofi M, et al. Characterization Studies of Biopolymeric Matrix and Cellulose Fibres Based Composites Related to Functionalized Fibre-Matrix Interface. In Interfaces in Particle and Fibre Reinforced Composites: Current Perspectives on Polymer, Ceramic, Metal and Extracellular Matrices; Woodhead Publishing: Sawston, UK, 2019.
21.
Getme AS, Patel B. A Review: Bio-Fiber’s as Reinforcement in Composites of Polylactic Acid (PLA). Mater. Today Proc. 2019, 26, 2116–2122. [Google Scholar]
22.
Vinod A, Sanjay MR, Suchart S, Jyotishkumar P. Renewable and Sustainable Biobased Materials: An Assessment on Biofibers, Biofilms, Biopolymers and Biocomposites. J. Clean. Prod. 2020, 258, 120978. [Google Scholar]
23.
Bari E, Morrell JJ, Sistani A. Durability of Natural/Synthetic/Biomass Fiber-Based Polymeric Composites: Laboratory and Field Tests. In Durability and Life Prediction in Biocomposites, Fibre-Reinforced Composites and Hybrid Composites; Woodhead: Sawston, UK, 2018.
24.
Jawaid M, Abdul Khalil HPS. Cellulosic/Synthetic Fibre Reinforced Polymer Hybrid Composites: A Review. Carbohydr. Polym. 2011, 86, 1–18. [Google Scholar]
25.
Kumar S, Manna A, Dang R. A Review on Applications of Natural Fiber-Reinforced Composites (NFRCs). Mater. Today Proc. 2021, 50, 1632–1636. [Google Scholar]
26.
Monteiro SN, Lopes FPD, Ferreira AS, Nascimento DCO. Natural-Fiber Polymer-Matrix Composites: Cheaper, Tougher, and Environmentally Friendly. JOM 2009, 61, 17–22. [Google Scholar]
27.
Madhusudhana HK, Ruhi K, Anand RL, Venkatesha CS. Experimental Study on Fracture Toughness of Natural Fibres Reinforced Hybrid Composites. IOP Conf. Ser. Mater. Sci. Eng. 2018, 376, 12088. [Google Scholar]
28.
Namvar F, Jawaid M, Tahir PM, Mohamad R, Azizi S, Khodavandi A, et al. Potential Use of Plant Fibres and Their Composites for Biomedical Applications. BioResources 2014, 9, 5688–5706. [Google Scholar]
29.
Komuraiah A, Kumar NS, Prasad BD. Chemical Composition of Natural Fibers and Its Influence on Their Mechanical Properties. Mech. Compos. Mater. 2014, 50, 359–376. [Google Scholar]
30.
Khan MZR, Srivastava SK, Gupta MK. Tensile and Flexural Properties of Natural Fiber Reinforced Polymer Composites: A Review. J. Reinf. Plast. Compos. 2018, 37, 1435–1455. [Google Scholar]
31.
Johnson R. Hemp as an Agricultural Commodity; Congressional Research Service: Washington, DC, USA, 2017.
32.
Radoor S, Jayakumar A, Siengchin S, Karayil J, Shivanna JM, Parameswaranpillai J. Cotton Fibers, Their Composites and Applications. In Plant Fibers, Their Composites, and Applications; Woodhead Publishing: Sawston, UK, 2022.
33.
Xu LL, Guo MX, Liu S, Bian SW. Graphene/Cotton Composite Fabrics as Flexible Electrode Materials for Electrochemical Capacitors. RSC Adv. 2015, 5, 25244–25249. [Google Scholar]
34.
Ku H, Wang H, Pattarachaiyakoop N, Trada M. A Review on the Tensile Properties of Natural Fiber Reinforced Polymer Composites. Compos. Part B Eng. 2011, 42, 856–873. [Google Scholar]
35.
West African Kenaf (Hibiscus Cannabinus L.) Natural Fiber Composite for Application in Automotive Industry. Available online: https://www.researchgate.net/publication/329979918_West_African_kenaf_Hibiscus_Cannabinus_L_natural_fiber_composite_for_application_in_automotive_industry (accessed on 17 July 2023).
36.
Holbery J, Houston D. Natural-Fiber-Reinforced Polymer Composites in Automotive Applications. JOM 2006, 58, 80–86. [Google Scholar]
37.
Indran S, Edwin Raj R, Sreenivasan VS. Characterization of New Natural Cellulosic Fiber from Cissus quadrangularis Root. Carbohydr. Polym. 2014, 110, 423–429. [Google Scholar]
38.
Wang H, Memon H, Hassan EAM, Miah MS, Ali MA. Effect of Jute Fiber Modification on Mechanical Properties of Jute Fiber Composite. Materials 2019, 12, 1226. [Google Scholar]
39.
Dixit S, Goel R, Dubey A, Shivhare PR, Bhalavi T. Natural Fibre Reinforced Polymer Composite Materials - A Review. Polym. Renew. Resour. 2017, 8, 71–78. [Google Scholar]
40.
Chin SC, Tee KF, Tong FS, Ong HR, Gimbun J. Thermal and Mechanical Properties of Bamboo Fiber Reinforced Composites. Mater. Today Commun. 2020, 23, 100876. [Google Scholar]
41.
Gao X, Zhu D, Fan S, Rahman MZ, Guo S, Chen F. Structural and Mechanical Properties of Bamboo Fiber Bundle and Fiber/Bundle Reinforced Composites: A Review. J. Mater. Res. Technol. 2022, 19, 1162–1190. [Google Scholar]
42.
Oushabi A. The Pull-out Behavior of Chemically Treated Lignocellulosic Fibers/Polymeric Matrix Interface (LF/PM): A Review. Compos. Part B Eng. 2019, 174, 107059. [Google Scholar]
43.
Živković I, Fragassa C, Pavlović A, Brugo T. Influence of Moisture Absorption on the Impact Properties of Flax, Basalt and Hybrid Flax/Basalt Fiber Reinforced Green Composites. Compos. Part B Eng. 2017, 111, 148–164. [Google Scholar]
44.
Wambua P, Ivens J, Verpoest I. Natural Fibres: Can They Replace Glass in Fibre Reinforced Plastics? Compos. Sci. Technol. 2003, 63, 1259–1264. [Google Scholar]
45.
Hamidon MH, Sultan MTH, Ariffin AH, Shah AUM. Effects of Fibre Treatment on Mechanical Properties of Kenaf Fibre Reinforced Composites: A Review. J. Mater. Res. Technol. 2019, 8, 3327–3337. [Google Scholar]
46.
Venkateshwaran N, Elaya Perumal A. Mechanical and Water Absorption Properties of Woven Jute/Banana Hybrid Composites. Fibers Polym. 2012, 13, 907–914. [Google Scholar]
47.
Sengupta S, Debnath S. Development of Sunnhemp (Crotalaria Juncea) Fibre Based Unconventional Fabric. Ind. Crops Prod. 2018, 116, 109–115. [Google Scholar]
48.
Latif R, Wakeel S, Khan NZ, Noor Siddiquee A, Lal Verma S, Akhtar Khan Z. Surface Treatments of Plant Fibers and Their Effects on Mechanical Properties of Fiber-Reinforced Composites: A Review. J. Reinf. Plas. Compos. 2019, 38, 15–30. [Google Scholar]
49.
Mathew L, Joseph R. Mechanical Properties of Short-Isora-Fiber-Reinforced Natural Rubber Composites: Effects of Fiber Length, Orientation, and Loading; Alkali Treatment; and Bonding Agent. J. Appl. Polym. Sci. 2007, 103, 25065. [Google Scholar]
50.
Pickering KL, Efendy MGA, Le TM. A Review of Recent Developments in Natural Fibre Composites and Their Mechanical Performance. Compos. Part A Appl. Sci. Manuf. 2016, 83, 98–112. [Google Scholar]
51.
Jayamaui E, Rahman MR, Benhur DA, Bakri MK, Kakair A, Khan A. Comparative Study of Fly Ash/Sugarcane Fiber Reinforced Polymer Composites Properties. BioResources 2020, 15, 5514–5531. [Google Scholar]
52.
Shrigandhi GD, Kothavale BS. Biodegradable Composites for Filament Winding Process. Mater. Today Proc. 2021, 42, 2762–2768. [Google Scholar]
53.
Cazaurang‐Martinez MN, Herrera‐Franco PJ, Gonzalez‐Chi PI, Aguilar‐Vega M. Physical and Mechanical Properties of Henequen Fibers. J. Appl. Polym. Sci. 1991, 43, 749–756. [Google Scholar]
54.
Pannu AS, Singh S, Dhawan V. Effect of Alkaline Treatment on Mechanical Properties of Biodegradable Composite (BF/PLA) Rod. Mater. Today Proc. 2021, 46, 9367–9371. [Google Scholar]
55.
Lo Re G, Spinella S, Boujemaoui A, Vilaseca F, Larsson PT, Adås F, et al. Poly(ϵ-Caprolactone) Biocomposites Based on Acetylated Cellulose Fibers and Wet Compounding for Improved Mechanical Performance. ACS Sustain. Chem. Eng. 2018, 6, 6753–6760. [Google Scholar]
56.
Abdullah NSE, Salim N, Roslan R. Investigation on the Effect of Alkaline Treatment on Seaweed/Polypropylene (SW/PP) Blend Composites. Mater. Today Proc. 2022, 51, 1362–1366. [Google Scholar]
57.
Tran TPT, Bénézet JC, Bergeret A. Rice and Einkorn Wheat Husks Reinforced Poly(Lactic Acid) (PLA) Biocomposites: Effects of Alkaline and Silane Surface Treatments of Husks. Ind. Crops Prod. 2014, 58, 111–124. [Google Scholar]
58.
Zaman HU, Khan RA. Acetylation Used for Natural Fiber/Polymer Composites. J. Thermoplast. Compos. Mater. 2021, 34, 3–23. [Google Scholar]
59.
Kivade SB, Gunge A, Nagamadhu M, Rajole S. Mechanical and Dynamic Mechanical Behavior of Acetylation-Treated Plain Woven Banana Reinforced Biodegradable Composites. Adv. Compos. Hybrid Mater. 2022, 5, 144–158. [Google Scholar]
60.
Mittal V, Saini R, Sinha S. Natural Fiber-Mediated Epoxy Composites – A Review. Compos. Part B Eng. 2016, 99, 425–435. [Google Scholar]
61.
Izwan SM, Sapuan SM, Zuhri MYM, Mohamed AR. Thermal Stability and Dynamic Mechanical Analysis of Benzoylation Treated Sugar Palm/Kenaf Fiber Reinforced Polypropylene Hybrid Composites. Polymers 2021, 13, 2961. [Google Scholar]
62.
Dehouche N, Idres C, Kaci M, Zembouai I, Bruzaud S. Effects of Various Surface Treatments on Aloe vera Fibers Used as Reinforcement in Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) (PHBHHx) Biocomposites. Polym. Degrad. Stab. 2020, 175, 109131. [Google Scholar]
63.
Rong MZ, Zhang MQ, Liu Y, Yang GC, Zeng HM. The Effect of Fiber Treatment on the Mechanical Properties of Unidirectional Sisal-Reinforced Epoxy Composites. Compos. Sci. Technol. 2001, 61, 1437–1447. [Google Scholar]
64.
Daghigh V, Lacy TE, Pittman CU, Daghigh H. Influence of Maleated Polypropylene Coupling Agent on Mechanical and Thermal Behavior of Latania Fiber-Reinforced PP/EPDM Composites. Polym. Compos. 2018, 39, E1751–E1759. [Google Scholar]
65.
Chun KS, Husseinsyah S, Osman H. Utilization of Cocoa Pod Husk as Filler in Polypropylene Biocomposites: Effect of Maleated Polypropylene. J. Thermoplast. Compos. Mater. 2015, 28, 1507–1521. [Google Scholar]
66.
Rabhi S, Benghanem N, Abdi S. Comparative Study of Two Biocomposites: Effect of Date Stone Flour Treated with Potassium Permanganate as a Filler on the Morphological and Elastic Properties. J. Compos. Mater. 2022, 56, 1071–1089. [Google Scholar]
67.
George G, Jose ET, Jayanarayanan K, Nagarajan ER, Skrifvars M, Joseph K.
Novel Bio-Commingled Composites Based on Jute/Polypropylene Yarns: Effect of Chemical Treatments on the Mechanical Properties. Compos. Part A Appl. Sci. Manuf. 2012, 43, 219–230. [Google Scholar]
68.
Li X, Tabil LG, Panigrahi S. Chemical Treatments of Natural Fiber for Use in Natural Fiber-Reinforced Composites: A Review. J. Polym. Environ. 2007, 15, 25–33. [Google Scholar]
69.
Sabri MNIM, Bakar MBA, Masri MN, Mohamed M, Noriman NZ, Dahham OS, et al. Effect of Chemical Treatment on Mechanical and Physical Properties of Non-Woven Kenaf Fiber Mat Reinforced Polypropylene Biocomposites. AIP Conf. Proc. 2020, 2213, 20262. [Google Scholar]
70.
Gulati D, Sain M. Fungal-Modification of Natural Fibers: A Novel Method of Treating Natural Fibers for Composite Reinforcement. J. Polym. Environ. 2006, 14, 347–352. [Google Scholar]
71.
Vilaseca F, Corrales F, Llop MF, Pelach MA, Mutjé P. Chemical Treatment for Improving Wettability of Biofibres into Thermoplastic Matrices. Compos. Interfaces 2005, 12, 725–738. [Google Scholar]
72.
Roy K, Debnath SC, Tzounis L, Pongwisuthiruchte A, Potiyaraj P. Effect of Various Surface Treatments on the Performance of Jute Fibers Filled Natural Rubber (NR) Composites. Polymers 2020, 12, 369. [Google Scholar]
73.
Satyanarayana KG, Arizaga GGC, Wypych F. Biodegradable Composites Based on Lignocellulosic Fibers—An Overview. Prog. Polym. Sci. 2009, 34, 982–1021. [Google Scholar]
74.
Bhagabati P. Biopolymers and Biocomposites-Mediated Sustainable High-Performance Materials for Automobile Applications. In Sustainable Nanocellulose and Nanohydrogels from Natural Sources; Elsevier: Oxford, UK, 2020; pp. 197–216.
75.
Calori IR, Braga G, de Jesus PdCC, Bi H, Tedesco AC. Polymer Scaffolds as Drug Delivery Systems. Eur. Polym. J. 2020, 129, 109621. [Google Scholar]
76.
George A, Sanjay MR, Srisuk R, Parameswaranpillai J, Siengchin S. A Comprehensive Review on Chemical Properties and Applications of Biopolymers and Their Composites. Int. J. Biol. Macromol. 2020, 154, 329–338. [Google Scholar]
77.
Christian SJ. Natural Fibre-Reinforced Noncementitious Composites (Biocomposites). In Nonconventional and Vernacular Construction Materials: Characterisation, Properties and Applications; Woodhead Publishing: Sawston, UK, 2019.
78.
Nair NR, Sekhar VC, Nampoothiri KM, Pandey A. Biodegradation of Biopolymers. In Current Developments in Biotechnology and Bioengineering: Production, Isolation and Purification of Industrial Products; Elsevier: Oxford, UK, 2017, 739–755.
79.
Mohamed SAN, Zainudin ES, Sapuan SM, Azaman MD, Arifin AMT. Introduction to Natural Fiber Reinforced Vinyl Ester and Vinyl Polymer Composites. In Natural Fiber Reinforced Vinyl Ester and Vinyl Polymer Composites: Development, Characterization and Applications; Woodhead Publishing: Sawston, UK, 2018.
80.
Ramesh M, Muthukrishnan M. 25—Biodegradable Polymer Blends and Composites for Food-Packaging Applications. In Biodegradable Polymers, Blends and Composites; Woodhead Publishing: Sawston, UK, 2022; pp. 693–716.
81.
Ingrao C, Siracusa V. 14—Quality- and Sustainability-Related Issues Associated with Biopolymers for Food Packaging Applications: A Comprehensive Review. In Biodegradable and Biocompatible Polymer Composites; Woodhead Publishing: Sawston, UK, 2018; pp. 401–418.
82.
Zhou J, Wang B, Xu C, Xu YZ, Tan H, Zhang X, et al. Performance of Composite Materials by Wood Fiber/Polydopamine/Silver Modified PLA and the Antibacterial Property. J. Mater. Res. Technol. 2022, 18, 428–438. [Google Scholar]
83.
Deshmukh K, Basheer Ahamed M, Deshmukh RR, Khadheer Pasha SK, Bhagat PR, Chidambaram K. 3—Biopolymer Composites with High Dielectric Performance: Interface Engineering. In Biopolymer Composites in Electronics; Elsevier: Oxford, UK, 2017; pp. 27–128.
84.
Ingrao C, Tricase C, Cholewa-Wójcik A, Kawecka A, Rana R, Siracusa V. Polylactic Acid Trays for Fresh-Food Packaging: A Carbon Footprint Assessment. Sci. Total Environ. 2015, 537, 385–398. [Google Scholar]
85.
Juodeikiene G, Vidmantiene D, Basinskiene L, Cernauskas D, Bartkiene E, Cizeikiene D. Green Metrics for Sustainability of Biobased Lactic Acid from Starchy Biomass vs Chemical Synthesis. Catal. Today 2015, 239, 11–16. [Google Scholar]
86.
Zwawi M. A Review on Natural Fiber Bio-Composites, Surface Modifications and Applications. Molecules 2021, 26, 404. [Google Scholar]
87.
Koller M. Advances in Polyhydroxyalkanoate (PHA) Production, Volume 2. Bioengineering 2020, 7, 24. [Google Scholar]
88.
Elmowafy E, Abdal-Hay A, Skouras A, Tiboni M, Casettari L, Guarino V. Polyhydroxyalkanoate (PHA): Applications in Drug Delivery and Tissue Engineering. Expert Rev. Med. Devices 2019, 16, 467–482. [Google Scholar]
89.
Liu Y, Ahmed S, Sameen DE, Wang Y, Lu R, Dai J, et al. A Review of Cellulose and Its Derivatives in Biopolymer-Based for Food Packaging Application. Trends Food Sci. Technol. 2021, 112, 532–546. [Google Scholar]
90.
Shaghaleh H, Xu X, Wang S. Current Progress in Production of Biopolymeric Materials Based on Cellulose, Cellulose Nanofibers, and Cellulose Derivatives. RSC Adv. 2018, 8, 825–842. [Google Scholar]
91.
Ichwan M, Onyianta AJ, Trask RS, Etale A, Eichhorn SJ. Production and Characterization of Cellulose Nanocrystals of Different Allomorphs from Oil Palm Empty Fruit Bunches for Enhancing Composite Interlaminar Fracture Toughness. Carbohydr. Polym. Technol. Appl. 2023, 5, 100272. [Google Scholar]
92.
Małachowska E, Dubowik M, Lipkiewicz A, Przybysz K, Przybysz P. Analysis of Cellulose Pulp Characteristics and Processing Parameters for Efficient Paper Production. Sustainability 2020, 12, 7219. [Google Scholar]
93.
Karandikar S, Mirani A, Waybhase V, Patravale VB, Patankar S. Nanovaccines for Oral Delivery-Formulation Strategies and Challenges. In Nanostructures for Oral Medicine; Elsevier: Oxford, UK, 2017; pp. 263–293.
94.
Zheng S, Bellido-Aguilar DA, Hu J, Huang Y, Zhao X, Wang Z, et al. Waterborne Bio-Based Epoxy Coatings for the Corrosion Protection of Metallic Substrates. Progr. Org. Coat. 2019, 136, 105265. [Google Scholar]
95.
Wang H, Hassan EAM, Memon H, Elagib THH, AllaIdris FA. Characterization of Natural Composites Fabricated from Abutilon-Fiber-Reinforced Poly (Lactic Acid). Processes 2019, 7, 583. [Google Scholar]
96.
Terry JS, Taylor AC. The Properties and Suitability of Commercial Bio‐based Epoxies for Use in Fiber‐reinforced Composites. J. Appl. Polym. Sci. 2021, 138, 50417. [Google Scholar]
97.
Aliotta L, Seggiani M, Lazzeri A, Gigante V, Cinelli P. A Brief Review of Poly (Butylene Succinate) (PBS) and Its Main Copolymers: Synthesis, Blends, Composites, Biodegradability, and Applications. Polymers 2022, 14, 844. [Google Scholar]
98.
Mondal MIH, Islam MM, Haque MI, Ahmed F. Natural, Biodegradable, Biocompatible and Bioresorbable Medical Textile Materials. In Medical Textiles from Natural Resources; Woodhead Publishing: Sawston, UK, 2022; pp. 87–116.
99.
Deepa C, Ramesh M. Biocomposites for Prosthesis. In Green Biocomposites for Biomedical Engineering: Design, Properties, and Applications; Woodhead Publishing: Sawston, UK, 2021; pp. 339–351.
100.
Abdulkhani A, Echresh Z, Allahdadi M. Effect of Nanofibers on the Structure and Properties of Biocomposites. In Fiber-Reinforced Nanocomposites: Fundamentals and Applications; Elsevier: Oxford, UK, 2020; pp. 321–357.
101.
Prakash SO, Sahu P, Madhan M, Johnson Santhosh A. A Review on Natural Fibre-Reinforced Biopolymer Composites: Properties and Applications. Int. J. Polym. Sci. 2022, 2022, 7820731. [Google Scholar]
102.
Tekinalp HL, Meng X, Lu Y, Kunc V, Love LJ, Peter WH, et al. High Modulus Biocomposites via Additive Manufacturing: Cellulose Nanofibril Networks as “Microsponges”. Compos. Part B Eng. 2019, 173, 106817. [Google Scholar]
103.
Baghaei B, Skrifvars M. All-Cellulose Composites: A Review of Recent Studies on Structure, Properties and Applications. Molecules 2020, 25, 2836. [Google Scholar]
104.
Soykeabkaew N, Nishino T, Peijs T. All-Cellulose Composites of Regenerated Cellulose Fibres by Surface Selective Dissolution. Compos. Part A Appl. Sci. Manuf. 2009, 40, 321–328. [Google Scholar]
105.
Mofokeng JP, Luyt AS, Tábi T, Kovács J. Comparison of Injection Moulded, Natural Fibre-Reinforced Composites with PP and PLA as Matrices. J. Thermoplast. Compos. Mater. 2012, 25, 927–948. [Google Scholar]
106.
Maiti P, Nandi AK. Influence of Chain Structure on the Miscibility of Poly(Vinylidene Fluoride) with Poly(Methyl Acrylate). Macromolecules 1995, 28, 8511–8516. [Google Scholar]
107.
Ilyas RA, Zuhri MYM, Norrrahim MNF, Misenan MSM, Jenol MA, Samsudin SA, et al. Natural Fiber-Reinforced Polycaprolactone Green and Hybrid Biocomposites for Various Advanced Applications. Polymers 2022, 14, 182. [Google Scholar]
108.
Na H, Huang J, Xu H, Liu F, Xie L, Zhu B, et al. Structure and Properties of PLA Composite Enhanced with Biomass Fillers from Herbaceous Plants. J. Renew. Mater. 2023, 11, 491–503. [Google Scholar]
109.
Solechan S, Suprihanto A, Widyanto SA, Triyono J, Fitriyana DF, Siregar JP, et al. Characterization of PLA/PCL/Nano-Hydroxyapatite (NHA) Biocomposites Prepared via Cold Isostatic Pressing. Polymers 2023, 15, 559. [Google Scholar]
110.
Ruz-Cruz MA, Herrera-Franco PJ, Flores-Johnson EA, Moreno-Chulim MV, Galera-Manzano LM, Valadez-González A. Thermal and Mechanical Properties of PLA-Based Multiscale Cellulosic Biocomposites. J. Mater. Res. Technol. 2022, 18, 485–495. [Google Scholar]
111.
Siqueira DD, Luna CBB, Ferreira ESB, Araújo EM, Wellen RMR. Tailored PCL/Macaíba Fiber to Reach Sustainable Biocomposites. J. Mater. Res. Technol. 2020, 9, 9691–9708. [Google Scholar]
112.
Rath A, Grisin B, Pallicity TD, Glaser L, Guhathakurta J, Oehlsen N, et al. Fabrication of Chitosan-Flax Composites with Differing Molecular Weights and Its Effect on Mechanical Properties. Compos. Sci. Technol. 2023, 235, 109952. [Google Scholar]
113.
de Oliveira Júnior JN, Lopes FPD, Simonassi NT, Lopera HAC, Monteiro SN, Vieira CMF. Ecofriendly Panels for Building with Eucalyptus Sawdust and Vegetal Polyurethane Resin: A Mechanical Evaluation. Case Stud. Constr. Mater. 2023, 18, e01839. [Google Scholar]
114.
Shiroud Heidari B, Muiños Lopez E, Harrington E, Ruan R, Chen P, Davachi SM, et al. Novel Hybrid Biocomposites for Tendon Grafts: The Addition of Silk to Polydioxanone and Poly(Lactide-Co-Caprolactone) Enhances Material Properties, in Vitro and in Vivo Biocompatibility. Bioact. Mater. 2023, 25, 291–306. [Google Scholar]
115.
Okubo K, Fujii T, Thostenson ET. Multi-Scale Hybrid Biocomposite: Processing and Mechanical Characterization of Bamboo Fiber Reinforced PLA with Microfibrillated Cellulose. Compos. Part A Appl. Sci. Manuf. 2009, 40, 469–475. [Google Scholar]
116.
Ceraulo M, La Mantia FP, Mistretta MC, Titone V. The Use of Waste Hazelnut Shells as a Reinforcement in the Development of Green Biocomposites. Polymers 2022, 14, 2151. [Google Scholar]
117.
Ho MP, Wang H, Lee JH, Ho CK, Lau KT, Leng J, et al. Critical Factors on Manufacturing Processes of Natural Fibre Composites. Compos. Part B Eng. 2012, 43, 3549–3562. [Google Scholar]
118.
Raji M, Abdellaoui H, Essabir H, Kakou C-A, Bouhfid R, el kacem Qaiss A. 3—Prediction of the Cyclic Durability of Woven-Hybrid Composites. In Durability and Life Prediction in Biocomposites, Fibre-Reinforced Composites and Hybrid Composites; Woodhead Publishing: Sawston, UK, 2019; pp. 27–62.
119.
Wu Y, Xia C, Cai L, Garcia AC, Shi SQ. Development of Natural Fiber-Reinforced Composite with Comparable Mechanical Properties and Reduced Energy Consumption and Environmental Impacts for Replacing Automotive Glass-Fiber Sheet Molding Compound. J. Clean. Prod. 2018, 184, 92–100. [Google Scholar]
120.
Domone P, Illston J. (Eds.) Manufacturing Techniques for Polymer Composites Used in Construction. In Construction Materials: Their Nature and Behaviour, 4th ed.; CRC Press: Boca Raton, FL, USA, 2018.
121.
Gupta MK, Singh R. PLA-Coated Sisal Fibre-Reinforced Polyester Composite: Water Absorption, Static and Dynamic Mechanical Properties. J. Compos. Mater. 2019, 53, 65–72. [Google Scholar]
122.
Sukmawan R, Takagi H, Nakagaito AN. Strength Evaluation of Cross-Ply Green Composite Laminates Reinforced by Bamboo Fiber. Compos. Part B Eng. 2016, 84, 9–16. [Google Scholar]
123.
Azlin MNM, Sapuan SM, Zuhri MYM, Zainudin ES. Effect of Stacking Sequence and Fiber Content on Mechanical and Morphological Properties of Woven Kenaf/Polyester Fiber Reinforced Polylactic Acid (PLA) Hybrid Laminated Composites. J. Mater. Res. Technol. 2022, 16, 1190–1201. [Google Scholar]
124.
Anbukarasu P, Sauvageau D, Elias A. The Effects of Solvent Casting Temperature and Physical Aging on Polyhydroxybutyrate-Graphene Nanoplatelet Composites. Polym. Compos. 2021, 42, 1451–1461. [Google Scholar]
125.
Kong I, Tshai KY, Hoque ME. Manufacturing of Natural Fibre-Reinforced Polymer Composites by Solvent Casting Method. In Manufacturing of Natural Fibre Reinforced Polymer Composites; Springer: Berlin/Heidelberg, Germany, 2015; pp. 331–349.
126.
Khan MA, Hussain Z, Liaqat U, Liaqat MA, Zahoor M. Preparation of Pbs/Plla/Hap Composites by the Solution Casting Method: Mechanical Properties and Biocompatibility. Nanomaterials 2020, 10, 1778. [Google Scholar]
127.
Kale RD, Gorade VG, Parmaj O. Development and Characterization Study of Silk Filament Reinforced Chitosan Biocomposite. J. Nat. Fibers 2020, 17, 1465878. [Google Scholar]
128.
Liu DY, Yuan XW, Bhattacharyya D, Easteal AJ. Characterisation of Solution Cast Cellulose Nanofibre - Reinforced Poly(Lactic Acid). Express Polym. Lett. 2010, 4, 26–31. [Google Scholar]
129.
Oyeoka HC, Ewulonu CM, Nwuzor IC, Obele CM, Nwabanne JT. Packaging and Degradability Properties of Polyvinyl Alcohol/Gelatin Nanocomposite Films Filled Water Hyacinth Cellulose Nanocrystals. J. Bioresour. Bioprod. 2021, 6, 168–185. [Google Scholar]
130.
Yokesahachart C, Yoksan R, Khanoonkon N, Mohanty AK, Misra M. Effect of Jute Fibers on Morphological Characteristics and Properties of Thermoplastic Starch/Biodegradable Polyester Blend. Cellulose 2021, 28, 5513–5530. [Google Scholar]
131.
Vandi LJ, Chan CM, Werker A, Richardson D, Laycock B, Pratt S. Experimental Data for Extrusion Processing and Tensile Properties of Poly(Hydroxybutyrate-Co-Hydroxyvalerate) (PHBV) Polymer and Wood Fibre Reinforced PHBV Biocomposites. Data Brief 2019, 22, 687–692. [Google Scholar]
132.
Gupta A, Chudasama B, Chang BP, Mekonnen T. Robust and Sustainable PBAT—Hemp Residue Biocomposites: Reactive Extrusion Compatibilization and Fabrication. Compos. Sci. Technol. 2021, 215, 109014. [Google Scholar]
133.
Rabbi MS, Islam T, Islam GMS. Injection-Molded Natural Fiber-Reinforced Polymer Composites–a Review. Int. J. Mech. Mater. Eng. 2021, 16, 15. [Google Scholar]
134.
Agüero Á, Garcia-Sanoguera D, Lascano D, Rojas-Lema S, Ivorra-Martinez J, Fenollar O, et al. Evaluation of Different Compatibilization Strategies to Improve the Performance of Injection-Molded Green Composite Pieces Made of Polylactide Reinforced with Short Flaxseed Fibers. Polymers 2020, 12, 0821. [Google Scholar]
135.
Shaharuddin SIS, Salit MS, Zainudin ES. A Review of the Effect of Moulding Parameters on the Performance of Polymeric Composite Injection Moulding. Turk. J. Eng. Environ. Sci. 2006, 30, 23–34. [Google Scholar]
136.
Bax B, Müssig J. Impact and Tensile Properties of PLA/Cordenka and PLA/Flax Composites. Compos. Sci. Technol. 2008, 68, 1601–1607. [Google Scholar]
137.
Huda MS, Mohanty AK, Drzal LT, Schut E, Misra M. “Green” Composites from Recycled Cellulose and Poly(Lactic Acid): Physico-Mechanical and Morphological Properties Evaluation. J. Mater. Sci. 2005, 40, 4221–4229. [Google Scholar]
138.
Verma N, Singh MK, Zafar S, Pathak H. Comparative Study of In-Situ Temperature Measurement during Microwave-Assisted Compression-Molding and Conventionally Compression-Molding Process. CIRP J. Manuf. Sci. Technol. 2021, 35, 336–345. [Google Scholar]
139.
Asgher M, Ahmad Z, Iqbal HMN. Bacterial Cellulose-Assisted de-Lignified Wheat Straw-PVA Based Bio-Composites with Novel Characteristics. Carbohydr. Polym. 2017, 161, 244–252. [Google Scholar]
140.
Ochi S. Mechanical Properties of Kenaf Fibers and Kenaf/PLA Composites. Mech. Mater. 2008, 40, 446–452. [Google Scholar]
141.
Porras A, Maranon A. Development and Characterization of a Laminate Composite Material from Polylactic Acid (PLA) and Woven Bamboo Fabric. Compos. Part B Eng. 2012, 43, 2782–2788. [Google Scholar]
142.
Nanthananon P, Seadan M, Pivsa-Art S, Hiroyuki H, Suttiruengwong S. Biodegradable Polyesters Reinforced with Eucalyptus Fiber: Effect of Reactive Agents. AIP Conf. Proc. 2017, 1914, 070012. [Google Scholar]
143.
Cuinat-Guerraz N, Dumont MJ, Hubert P. Environmental Resistance of Flax/Bio-Based Epoxy and Flax/Polyurethane Composites Manufactured by Resin Transfer Moulding. In Proceedings of 20th International Conference on Composite Materials, Copenhagen, Denmark, 19–24 July 2015.
144.
Tran P, Graiver D, Narayan R. Biocomposites Synthesized from Chemically Modified Soy Oil and Biofibers. J. Appl. Polym. Sci. 2006, 102, 69–75. [Google Scholar]
145.
Reinhardt M, Kaufmann J, Kausch M, Kroll L. PLA-Viscose-Composites with Continuous Fibre Reinforcement for Structural Applications. Procedia Mater. Sci. 2013, 2, 137–143. [Google Scholar]
146.
Baghaei B, Skrifvars M, Berglin L. Characterization of Thermoplastic Natural Fibre Composites Made from Woven Hybrid Yarn Prepregs with Different Weave Pattern. Compos. Part A Appl. Sci. Manuf. 2015, 76, 154–161. [Google Scholar]
147.
Mindermann P, Pérez MG, Knippers J, Gresser GT. Investigation of the Fabrication Suitability, Structural Performance, and Sustainability of Natural Fibers in Coreless Filament Winding. Materials 2022, 15, 3260. [Google Scholar]
148.
Tripathi S, Mandal SS, Bauri S, Maiti P. 3D Bioprinting and Its Innovative Approach for Biomedical Applications. MedComm 2023, 4, e194. [Google Scholar]
149.
Zhang W, Wang C, Gu S, Yu H, Cheng H, Wang G. Physical-Mechanical Properties of Bamboo Fiber Composites Using Filament Winding. Polymers 2021, 13, 2913. [Google Scholar]
150.
Balla VK, Kate KH, Satyavolu J, Singh P, Tadimeti JGD. Additive Manufacturing of Natural Fiber Reinforced Polymer Composites: Processing and Prospects. Compos. Part B Eng. 2019, 17, 106956. [Google Scholar]
151.
Arrigo R, Frache A. FDM Printability of PLA Based-Materials: The Key Role of the Rheological Behavior. Polymers 2022, 14, 1754. [Google Scholar]
152.
Bhagia S, Bornani K, Agarwal R, Satlewal A, Ďurkovič J, Lagaňa R, et al. Critical Review of FDM 3D Printing of PLA Biocomposites Filled with Biomass Resources, Characterization, Biodegradability, Upcycling and Opportunities for Biorefineries. Appl. Mater. Today 2021, 24, 101078. [Google Scholar]
153.
Niang B, Schiavone N, Askanian H, Verney V, Ndiaye D, Diop AB. Development and Characterization of PBSA-Based Green Composites in 3D-Printing by Fused Deposition Modelling. Materials 2022, 15, 7570. [Google Scholar]
154.
Le Duigou A, Correa D, Ueda M, Matsuzaki R, Castro M. A Review of 3D and 4D Printing of Natural Fibre Biocomposites. Mater. Des. 2020, 194, 108911. [Google Scholar]
155.
Depuydt D, Balthazar M, Hendrickx K, Six W, Ferraris E, Desplentere F, et al. Production and Characterization of Bamboo and Flax Fiber Reinforced Polylactic Acid Filaments for Fused Deposition Modeling (FDM). Polym. Compos. 2019, 40, 1951–1963. [Google Scholar]
156.
Agaliotis EM, Ake-Concha BD, May-Pat A, Morales-Arias JP, Bernal C, Valadez-Gonzalez A, et al. Tensile Behavior of 3D Printed Polylactic Acid (PLA) Based Composites Reinforced with Natural Fiber. Polymers 2022, 14, 3976. [Google Scholar]
157.
Park JB, Lakes RS. (Eds.) Characterization of Materials—I. In Biomaterials; Springer: New York, NY, USA, 2007; pp. 41–81.
158.
Cross JO, Opila RL, Boyd IW, Kaufmann EN. Materials Characterization and the Evolution of Materials. MRS Bull. 2015, 40, 1019–1034. [Google Scholar]
159.
Lin J, Yang Z, Hu X, Hong G, Zhang S, Song W. The Effect of Alkali Treatment on Properties of Dopamine Modification of Bamboo Fiber/Polylactic Acid Composites. Polymers 2018, 10, 403. [Google Scholar]
160.
Sanjay MR, Siengchin S, Parameswaranpillai J, Jawaid M, Pruncu CI, Khan A. A Comprehensive Review of Techniques for Natural Fibers as Reinforcement in Composites: Preparation, Processing and Characterization. Carbohydr. Polym. 2019, 207, 108–121. [Google Scholar]
161.
Zhu Z, Hao M, Zhang N. Influence of Contents of Chemical Compositions on the Mechanical Property of Sisal Fibers and Sisal Fibers Reinforced PLA Composites. J. Nat. Fibers 2020, 17, 101–112. [Google Scholar]
162.
Abraham E, Elbi PA, Deepa B, Jyotishkumar P, Pothen LA, Narine SS, Thomas S. X-Ray Diffraction and Biodegradation Analysis of Green Composites of Natural Rubber/Nanocellulose. Polym. Degrad. Stab. 2012, 97, 2378–2387. [Google Scholar]
163.
Lorwanishpaisarn N, Sae-Oui P, Amnuaypanich S, Siriwong C. Fabrication of Untreated and Silane-Treated Carboxylated Cellulose Nanocrystals and Their Reinforcement in Natural Rubber Biocomposites. Sci. Rep. 2023, 13, 2517. [Google Scholar]
164.
French AD. Idealized Powder Diffraction Patterns for Cellulose Polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar]
165.
Somseemee O, Saeoui P, Schevenels FT, Siriwong C. Enhanced Interfacial Interaction between Modified Cellulose Nanocrystals and Epoxidized Natural Rubber via Ultraviolet Irradiation. Sci. Rep. 2022, 12, 6682. [Google Scholar]
166.
Somseemee O, Sae-Oui P, Siriwong C. Reinforcement of Surface-Modified Cellulose Nanofibrils Extracted from Napier Grass Stem in Natural Rubber Composites. Ind. Crops Prod. 2021, 171, 113881. [Google Scholar]
167.
Jayamani E, Loong TG, Bakri MK. Bin Comparative Study of Fourier Transform Infrared Spectroscopy (FTIR) Analysis of Natural Fibres Treated with Chemical, Physical and Biological Methods. Polym. Bull. 2020, 77, 1605–1629. [Google Scholar]
168.
Smoca A. FTIR Spectroscopy Analysis of PLA Biocomposites Reinforced with Hemp Fibers. Key Eng. Mater. 2020, 850, 112–117. [Google Scholar]
169.
Chen L, Zhu JY, Baez C, Kitin P, Elder T. Highly Thermal-Stable and Functional Cellulose Nanocrystals and Nanofibrils Produced Using Fully Recyclable Organic Acids. Green Chem. 2016, 18, 3835–3843. [Google Scholar]
170.
Zhu B, Ma J, Wang J, Wu J, Peng D. Thermal, Dielectric and Compressive Properties of Hollow Glass Microsphere Filled Epoxy-Matrix Composites. J. Reinf. Plas. Compos. 2012, 31, 1311–1326. [Google Scholar]
171.
Satsum A, Busayaporn W, Rungswang W, Soontaranon S, Thumanu K, Wanapu C. Structural and Mechanical Properties of Biodegradable Poly(Lactic Acid) and Pectin Composites: Using Bionucleating Agent to Improve Crystallization Behavior. Polym. J. 2022, 54, 921–930. [Google Scholar]
172.
Bledzki AK, Jaszkiewicz A, Scherzer D. Mechanical Properties of PLA Composites with Man-Made Cellulose and Abaca Fibres. Compos. Part A Appl. Sci. Manuf. 2009, 40, 404–412. [Google Scholar]
173.
Muthuraj R, Misra M, Mohanty AK. Biocomposite Consisting of Miscanthus Fiber and Biodegradable Binary Blend Matrix: Compatibilization and Performance Evaluation. RSC Adv. 2017, 7, 27538–27548. [Google Scholar]
174.
Tengsuthiwat J, Yorseng K, Siengchin S, Parameswaranpillai J. Thermomechanical, Water Absorption, Ultraviolet Resistance and Laser-Assisted Electroless Plating Behavior of Cu2O and Melamine–Formaldehyde-Coated Sisal Fiber-Modified Poly(Lactic Acid) Composites. Polym. Compos. 2019, 40, 25182. [Google Scholar]
175.
Dobrosielska M, Dobrucka R, Kozera P, Brząkalski D, Gabriel E, Głowacka J, Jałbrzykowski M, Kurzydłowski KJ, Przekop RE. Beeswax as a Natural Alternative to Synthetic Waxes for Fabrication of PLA/Diatomaceous Earth Composites. Sci. Rep. 2023, 13, 1161. [Google Scholar]
176.
Bassyouni M. Dynamic Mechanical Properties and Characterization of Chemically Treated Sisal Fiber-Reinforced Polypropylene Biocomposites. J. Reinf. Plas. Compos. 2018, 37, 1402–1417. [Google Scholar]
177.
Manral A, Ahmad F, Chaudhary V. Static and Dynamic Mechanical Properties of PLA Bio-Composite with Hybrid Reinforcement of Flax and Jute. Mater. Today Proc. 2020, 25, 577–580. [Google Scholar]
178.
Neto JSS, Lima RAA, Cavalcanti DKK, Souza JPB, Aguiar RAA, Banea MD. Effect of Chemical Treatment on the Thermal Properties of Hybrid Natural Fiber-Reinforced Composites. J. Appl. Polym. Sci. 2019, 136, 47154. [Google Scholar]
179.
Saba N, Jawaid M, Sultan MT.H. An Overview of Mechanical and Physical Testing of Composite Materials. In Mechanical and Physical Testing of Biocomposites, Fibre-Reinforced Composites and Hybrid Composites; Woodhead Publishing: Sawston, UK, 2018; pp. 1–12.
180.
ASTM-D638-14
Standard Test Method for Tensile Properties of Plastics; ASTM Standards; ASTM: West
Conshohocken, PA, USA, 2014.
181.
ASTM D790-17 Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials. D790; ASTM Standards; ASTM: West Conshohocken, PA, USA, 2012.
182.
Gadzama SW, Sunmonu OK, Isiaku US, Danladi A. Effects of Surface Modifications on the Mechanical Properties of Reinforced Pineapple Leaf Fibre Polypropylene Composites. Adv. Chem. Eng. Sci. 2020, 10, 24–39. [Google Scholar]
183.
Fazal A, Fancey KS. Viscoelastically Prestressed Polymeric Matrix Composites – Effects of Test Span and Fibre Volume Fraction on Charpy Impact Characteristics. Compos. Part B Eng. 2013, 44, 472–479. [Google Scholar]
184.
Singh R, Davim JP. Additive Manufacturing: Applications and Innovations; CRC Press: Boca Raton, FL, USA, 2018.
185.
Ismail H, Edyham MR, Wirjosentono B. Bamboo Fibre Filled Natural Rubber Composites: The Effects of Filler Loading and Bonding Agent. Polym. Test. 2002, 21, 139–144. [Google Scholar]
186.
Nair SS, Chen H, Peng Y, Huang Y, Yan N. Polylactic Acid Biocomposites Reinforced with Nanocellulose Fibrils with High Lignin Content for Improved Mechanical, Thermal, and Barrier Properties. ACS Sustain. Chem. Eng. 2018, 6, 10058–10068. [Google Scholar]
187.
Farhat H. Materials and Coating Technologies. In Operation, Maintenance, and Repair of Land-Based Gas Turbines; Elsevier: Oxford, UK, 2021.
188.
Leterrier Y. Mechanics of Curvature and Strain in Flexible Organic Electronic Devices. In Handbook of Flexible Organic Electronics: Materials, Manufacturing and Applications; Woodhead Publishing: Sawston, UK, 2015.
189.
de Bilbao E, Soulat D, Hivet G, Launay J, Gasser A. Bending Test of Composite Reinforcements. Int. J. Mater. Form. 2008, 1, 835–838. [Google Scholar]
190.
Habibi M, Laperrière L. Combining Digital Image Correlation and Acoustic Emission to Characterize the Flexural Behavior of Flax Biocomposites. Appl. Mech. 2023, 4, 21. [Google Scholar]
191.
Noorunnisa Khanam P, Al-Maadeed MA, Naseema Khanam P. 8—Silk as a Reinforcement in Polymer Matrix Composites. In Advances in Silk Science and Technology; Woodhead Publishing: Sawston, UK, 2015; pp. 143–170.
192.
Dann T, Malbon C. Chapter Eight—Tearing or Ripping of Fabrics. In Forensic Textile Science; Woodhead Publishing: Sawston, UK, 2017; pp. 169–180.
193.
El Miri N, Abdelouahdi K, Zahouily M, Fihri A, Barakat A, Solhy A, et al. Bio-Nanocomposite Films Based on Cellulose Nanocrystals Filled Polyvinyl Alcohol/Chitosan Polymer Blend. J. Appl. Polymer Sci. 2015, 132, 42004. [Google Scholar]
194.
Trache D, Donnot A, Khimeche K, Benelmir R, Brosse N. Physico-Chemical Properties and Thermal Stability of Microcrystalline Cellulose Isolated from Alfa Fibres. Carbohydr. Polym. 2014, 104, 223–230. [Google Scholar]
195.
Nam S, Condon BD, Delhom CD, Fontenot KR. Silver-Cotton Nanocomposites: Nano-Design of Microfibrillar Structure Causes Morphological Changes and Increased Tenacity. Sci. Rep. 2016, 6, 37320. [Google Scholar]
196.
Joshi J, Homburg SV, Ehrmann A. Atomic Force Microscopy (AFM) on Biopolymers and Hydrogels for Biotechnological Applications—Possibilities and Limits. Polymers 2022, 14, 1267. [Google Scholar]
197.
Raj G, Balnois E, Baley C, Grohens Y. Interfacial Studies of Polylactic Acid (PLA)/Flax Biocomposite: From Model Surface to Fibre Treatment. In Proceedings of the 17th ICCM International Conferences on Composite Materials, Edinburgh, UK, 27–31 July 2009.
198.
Zlotnikov I, Zolotoyabko E, Fratzl P. Nano-Scale Modulus Mapping of Biological Composite Materials: Theory and Practice. Progr. Mater. Sci. 2017, 87, 292–320. [Google Scholar]
199.
Huang S, Wang X, Zhang Y, Meng Y, Hua F, Xia X. Cellulose Nanofibers/Polyvinyl Alcohol Blends as an Efficient Coating to Improve the Hydrophobic and Oleophobic Properties of Paper. Sci. Rep. 2022, 12, 16148. [Google Scholar]
200.
Mazzanti V, Mollica F. A Review of Wood Polymer Composites Rheology and Its Implications for Processing. Polymers 2020, 12, 2304. [Google Scholar]
201.
Zuhudi NZM, Fadzil FAM, Zulkifli M, Yahaya ANA, Nur NM, Rahman NAA, et al. A rheological study of fibre reinforced composites and the factors that affect rheological behaviour during impregnation process: a review. J. Adv. Res. Fluid Mech. Therm. Sci. 2022, 89, 167–181. [Google Scholar]
202.
Hodzic A. Re-Use, Recycling and Degradation of Composites. In Green Composites: Polymer Composites and the Environment; CRC Press: Boca Raton, FL, USA, 2004.
203.
Maiti P, Kumar S. Nanoparticle Induced Controlled Biodegradation of Polymers; In Environment Friendly Biodegradable Polymers: Present and Future; Mallick Book Centre: Kolkata, India, 2016.
204.
Ali SS, Elsamahy T, Al-Tohamy R, Zhu D, Mahmoud YAG, Koutra E, et al. Plastic Wastes Biodegradation: Mechanisms, Challenges and Future Prospects. Sci. Total Environ. 2021, 780, 146590. [Google Scholar]
205.
Lucas N, Bienaime C, Belloy C, Queneudec M, Silvestre F, Nava-Saucedo JE. Polymer Biodegradation: Mechanisms and Estimation Techniques - A Review. Chemosphere 2008, 73, 429–442. [Google Scholar]
206.
Ahmed T, Shahid M, Azeem F, Rasul I, Shah AA, Noman M, et al. Biodegradation of Plastics: Current Scenario and Future Prospects for Environmental Safety. Environ. Sci. Pollut. Res. 2018, 25, 7287–7298. [Google Scholar]
207.
Kumar S, Maiti P. Controlled Biodegradation of Polymers Using Nanoparticles and Its Application. RSC Adv. 2016, 6, 67449–67480. [Google Scholar]
208.
Kumar S, Maiti P. Understanding the Controlled Biodegradation of Polymers Using Nanoclays. Polymer 2015, 76, 25–33. [Google Scholar]
209.
Kumar S, Singh S, Senapati S, Singh AP, Ray B, Maiti P. Controlled Drug Release through Regulated Biodegradation of Poly(Lactic Acid) Using Inorganic Salts. Int. J. Biol. Macromol. 2017, 104, 487–497. [Google Scholar]
210.
Banerjee A, Chatterjee K, Madras G. Enzymatic Degradation of Polymers: A Brief Review. Mater. Sci. Technol. 2014, 30, 567–573. [Google Scholar]
211.
Zaaba NF, Jaafar M. A Review on Degradation Mechanisms of Polylactic Acid: Hydrolytic, Photodegradative, Microbial, and Enzymatic Degradation. Polym. Eng. Sci. 2020, 60, 2061–2075. [Google Scholar]
212.
Xu L, Crawford K, Gorman CB. Effects of Temperature and PH on the Degradation of Poly(Lactic Acid) Brushes. Macromolecules 2011, 44, 4777–4782. [Google Scholar]
213.
Hegyesi N, Zhang Y, Kohári A, Polyák P, Sui X, Pukánszky B. Enzymatic Degradation of PLA/Cellulose Nanocrystal Composites. Ind. Crops Prod. 2019, 141, 111799. [Google Scholar]
214.
Shi K, Ma Q, Su T, Wang Z. Preparation of Porous Materials by Selective Enzymatic Degradation: Effect of in Vitro Degradation and in Vivo Compatibility. Sci. Rep. 2020, 10, 7031. [Google Scholar]
215.
Vasile C, Pamfil D, Râpă M, Darie-Niţă RN, Mitelut AC, Popa EE, Popescu PA, Draghici MC, Popa ME. Study of the Soil Burial Degradation of Some PLA/CS Biocomposites. Compos. Part B Eng. 2018, 142, 251–262. [Google Scholar]
216.
Yaacob ND, Ismail H, Ting SS. Soil Burial of Polylactic Acid/Paddy Straw Powder Biocomposite. BioResources 2016, 11, 1255–1269. [Google Scholar]
217.
Slezak R, Krzystek L, Puchalski M, Krucińska I, Sitarski A. Degradation of Bio-Based Film Plastics in Soil under Natural Conditions. Sci. Total Environ. 2023, 866, 161401. [Google Scholar]
218.
Sankhla IS, Sharma G, Tak A. Fungal Degradation of Bioplastics: An Overview. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Oxford, UK, 2020; pp. 35–47.
219.
Stoleru E, Hitruc EG, Vasile C, Oprică L. Biodegradation of Poly(Lactic Acid)/Chitosan Stratified Composites in Presence of the Phanerochaete Chrysosporium Fungus. Polym. Degrad. Stab. 2017, 143, 118–129. [Google Scholar]
220.
Soutis C, Yi XS, Bachmann J. How green composite materials could benefit aircraft construction. Sci. China Technol. Sci. 2019, 62, 1478–1480. [Google Scholar]
221.
Akampumuza O, Wambua PM, Ahmed A, Li W, Qin XH. Review of the Applications of Biocomposites in the Automotive Industry. Polym. Compos. 2017, 38, 2553–2569. [Google Scholar]
222.
Li M, Pu Y, Thomas VM, Yoo CG, Ozcan S, Deng Y, et al. Recent Advancements of Plant-Based Natural Fiber–Reinforced Composites and Their Applications. Compos. Part B Eng. 2020, 200, 108254. [Google Scholar]
223.
Sathyaraj S, Dhas JER, Balakrishnan HK. Recent Developments of Fiber-Reinforced Polymer Composites in Automotive. In Fiber-Reinforced Polymer: Processes and Applications; Nova Science Publishers: Hauppauge, NY, USA, 2021.
224.
Awwad EA, Hamad B, Mabsout M, Khatib H. Sustainable Concrete Using Hemp Fibres. Proc. Inst. Civil Eng. Construct. Mater. 2013, 166, 45–53. [Google Scholar]
225.
Sáez-Pérez MP, Durán-Suárez JA, Castro-Gomes J. Improving the Behaviour of Green Concrete Geopolymers Using Different HEMP Preservation Conditions (Fresh and Wet). Minerals 2022, 12, 1530. [Google Scholar]
226.
Versino F, López OV, García MA. Green Biocomposites for Packaging Applications. In Biocomposite Materials. Composites Science and Technology; Springer, Singapore, 2021.
227.
Farias NC, Major I, Devine D, Brennan Fournet M, Pezzoli R, Farshbaf Taghinezhad S, et al. Multiple Recycling of a PLA/PHB Biopolymer Blend for Sustainable Packaging Applications: Rheology-Morphology, Thermal, and Mechanical Performance Analysis. Polym. Eng. Sci. 2022, 62, 25962. [Google Scholar]
228.
Koronis G, Silva A, Fontul M. Green Composites: A Review of Adequate Materials for Automotive Applications. Compos. Part B Eng. 2013, 44, 120–127. [Google Scholar]
229.
Ashori A. Wood-Plastic Composites as Promising Green-Composites for Automotive Industries! Bioresour. Technol. 2008, 99, 4661–4667. [Google Scholar]
230.
Mohanty AK, Misra M, Drzal LT. Sustainable Bio-Composites from Renewable Resources: Opportunities and Challenges in the Green Materials World. J. Polym. Environ. 2002, 10, 19–26. [Google Scholar]
231.
Fan J, Nassiopoulos E, Brighton J, De Larminat A, Njuguna J. New Structural Biocomposites for Car Applications. In Proceedings of the Society of Plastics Engineers-EUROTEC 2011 Conference Proceedings, Barcelona, Spain, 14–15 November 2011.
232.
Naseri N, Algan C, Jacobs V, John M, Oksman K, Mathew AP. Electrospun Chitosan-Based Nanocomposite Mats Reinforced with Chitin Nanocrystals for Wound Dressing. Carbohydr. Polym. 2014, 109, 7–15. [Google Scholar]
233.
Nagarwal RC, Singh PN, Kant S, Maiti P, Pandit JK. Chitosan Coated PLA Nanoparticles for Ophthalmic Delivery: Characterization, in-Vitro and in-Vivo Study in Rabbit Eye. J. Biomed. Nanotechnol. 2010, 6, 648–657. [Google Scholar]
234.
Luzi F, Puglia D, Torre L. Natural Fiber Biodegradable Composites and Nanocomposites: A Biomedical Application. In Biomass, Biopolymer-Based Materials, and Bioenergy: Construction, Biomedical, and other Industrial Applications; Woodhead Publishing: Sawston, UK, 2019.
235.
Erturk PA, Altuntas S, Irmak G, Buyukserin F. Bioinspired Collagen/GelatinNanopillared Films as a Potential Implant Coating Material. ACS Appl. Bio Mater. 2022, 5, 4913–4921. [Google Scholar]
236.
Javadian A, Smith IFC, Hebel DE. Application of Sustainable Bamboo-Based Composite Reinforcement in Structural-Concrete Beams: Design and Evaluation. Materials 2020, 13, 696. [Google Scholar]
237.
Asgher M, Qamar SA, Bilal M, Iqbal HMN. Bio-Based Active Food Packaging Materials: Sustainable Alternative to Conventional Petrochemical-Based Packaging Materials. Food Res. Int. 2020, 137, 109625. [Google Scholar]
238.
Dabrowska A. Plant-Oil-Based Fibre Composites for Boat Hulls. Materials 2022, 15, 1699. [Google Scholar]
239.
Mydin AO, Majeed SS, Omar R, Awoyera PO, Najm HM. Sustainable Lightweight Foamed Concrete Using Hemp Fibre for Mechanical Properties Improvement. J. Adv. Res. Appl. Mech. 2023, 101, 19–35. [Google Scholar]
240.
Avella M, Buzarovska A, Errico ME, Gentile G, Grozdanov A. Eco-Challenges of Bio-Based Polymer Composites. Materials 2009, 2, 911. [Google Scholar]
241.
Al-Oqla FM, Almagableh A, Omari MA. Design and Fabrication of Green Biocomposites. In Green Energy and Technology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 45–67.
242.
Akter M, Uddin MH, Tania IS. Biocomposites Based on Natural Fibers and Polymers: A Review on Properties and Potential Applications. J. Reinf. Plas. Compos. 2022, 41, 705–742. [Google Scholar]
243.
Yatigala NS, Bajwa DS, Bajwa SG. Compatibilization Improves Physico-Mechanical Properties of Biodegradable Biobased Polymer Composites. Compos. Part A Appl. Sci. Manuf. 2018, 107, 315–325. [Google Scholar]
244.
Rajan VS, Govindaraju M, Ramu M, Satheeshkumar V. Influence of Metal Foam Properties on Performance of Polymer Composite Spur Gear. Mater. Today Proc. 2020, 24, 1244–1250. [Google Scholar]