1. Introduction
Fontan circulation relies on passive return of venous flow into the pulmonary circulation from a superior and inferior pulmonary caval connection. Healthy Fontan circulation requires a healthy pulmonary vascular bed and low mean pulmonary artery pressures (mPAP) which are equivalent to Fontan pressures in an unobstructed circuit. The demands on the pulmonary circulation increase dramatically with physical activity in order to augment pulmonary blood flow and consequently cardiac output to meet the metabolic demands of exercise [
1]. Cardiopulmonary exercise testing (CPET) is useful in determining and monitoring exercise capacity in patients with Fontan circulation, and thus plays an important prognostic role [
2,
3]. Limited exercise tolerance is common amongst patients with Fontan circulation and may be caused by multiple factors including single ventricle dysfunction, atrio-ventricular regurgitation and elevated Fontan pressures [
1,
2,
4]. The accurate assessment of Fontan pressures during exercise is desirable however technically challenging. Some authors have utilized peripheral venous pressure measurement during exercise in adult patients with Fontan circulation [
5]. Recently, the implantable hemodynamic monitor (IHM, CardioMEMs
TM HF system, Abbott, Abbott Park, IL, USA) has become available for assessing mPAP and has been utilized for monitoring mPAP during exercise in adult patients with heart failure [
6]. The safety of IHM in adult patients with Fontan circulation has been demonstrated [
7,
8]. A previous study utilized IHM to monitor mPAP during six-minute walk test in adults [
9]. Our Fontan program started using IHM in 2021 and we have previously reported safety of this device in pediatric patients with Fontan circulation [
2]. We use IHM recordings for guiding treatment at home and during their clinic visits. We present a case series of pediatric patients with Fontan circulation who had undergone IHM placement for failing Fontan physiology. We report the utility of IHM in assessing changes in Fontan pressure with CPET in these patients.
2. Clinical Case Series
A retrospective review was performed and included patients with Fontan circulation and IHM who also underwent a clinical CPET. Institutional IRB approved the study. At our institute, starting September 2021, patients were selected for IHM implantation at the time of catheterization for close monitoring of Fontan related complications. The indications included evidence of advanced liver disease, severe protein losing enteropathy (PLE) or ventricular dysfunction requiring close medical management. Other considerations included distance from tertiary care center, lack of local health care resources and patients living at elevation. So far, 17 Fontan patients have undergone IHM implantation at our center. We present a case series of 4 patients post IHM who have performed a clinical CPET. The indications for IHM implantation are described in .
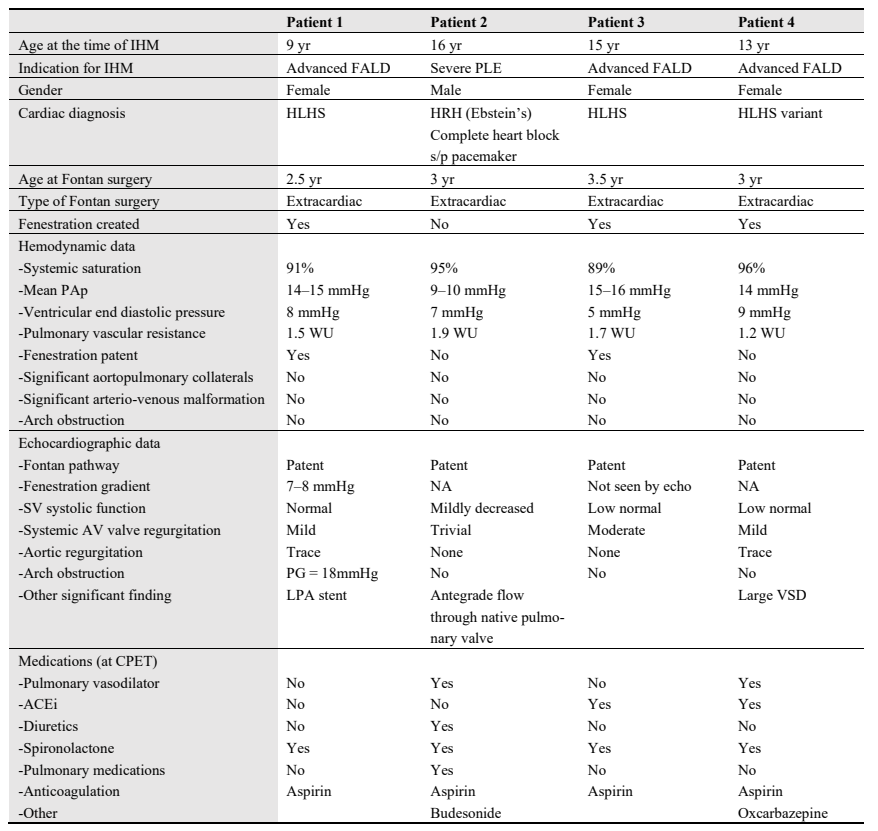
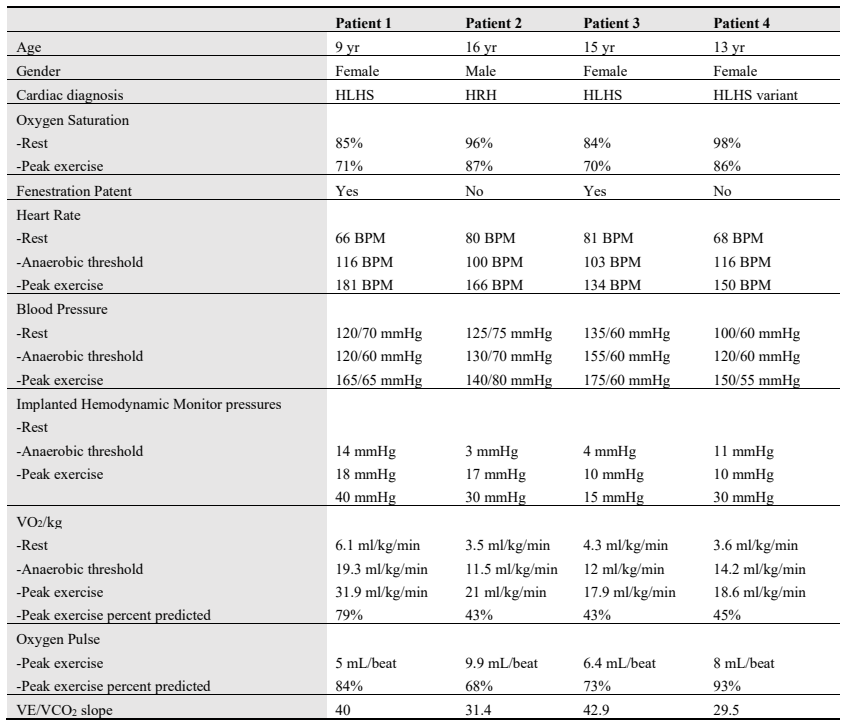
Patients performed exercise on an upright cycle ergometer using standardized ramp protocols with ramps set to achieve an exercise time of about 10 min. Electrocardiogram, blood pressure and oxygen saturation were measured continuously. Breath-by-breath metabolic data was obtained using a Vyaire Vyntus metabolic cart. IHM was interrogated using the hospital testing device. To assess orthostatic changes in Fontan pressures, we began with a supine measurement of mPAP to correlate with home measurements and then repeated a measurement when the patient was upright on the cycle ergometer. During active exercise, contact between the IHM and testing device was maintained consistently by an additional operator to ensure there is good signal strength for accurate measurements (). During IHM recording, the monitor displayed negative pressures related to the negative inspiratory pressure. We confirmed with IHM technical team that the monitor erroneously labels the patient’s respiratory rate during exercise as “heart rate” due to these negative inspiratory pressures (c). Similar to cardiac catheterization with a free breathing patient, end-expiratory pressures were recorded as the Fontan pressure or mPAP. The attending physician supervised the CPET and interpreted the IHM mPAP during exercise.
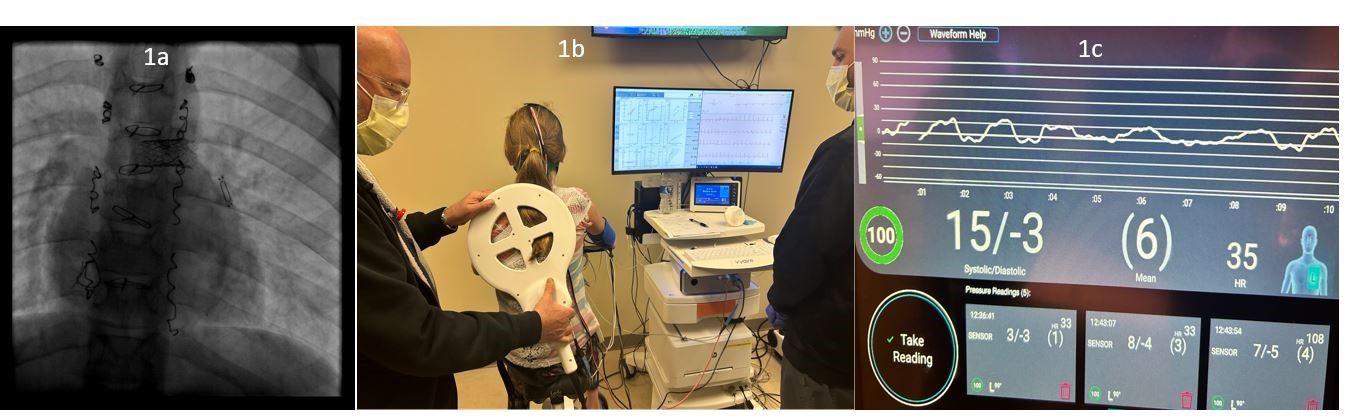
. <b>Interrogation of implanted hemodynamic monitor (CardioMEMs device) during exercise.</b> (<b>a</b>) shows the fluoroscopic view of the IHM implanted in distal left pulmonary artery in patient 1. (<b>b</b>) shows the IHM device being interrogated on the same patient during CPET. The testing device is being held in a stable position in close contact with the patient near the implantation location. (<b>c</b>) shows the IHM recording of the same patient during exercise. Note there is good communication between the interrogation device and the sensor, as indicated by the displayed signal strength of 100% in the (1C-lower left corner). Negative pressures are noted during higher negative inspiratory pressures due to patient’s breathing during exercise. The end-expiratory pressure (15 mmHg) represents the Fontan (mean PAp).
. Patient characteristics. Implanted hemoydnamic monitor (IHM), Fontan associated liver disease (FALD), Protein losing enteropathy (PLE) Hypoplastic left heart syndrome (HLHS), Hypoplastic right heart (HRH), Angiotensin converting enzyme inhibitor (ACEi).
shows the metabolic and hemodynamic data obtained on our patients during CPET. All patients achieved a respiratory exchange ratio > 1.1 and all but one had a low exercise capacity for Fontan circulation (). IHM recordings during different phases of exercise for each patient along with CPET data are presented in . Orthostatic changes were noted in mPAP and all patients showed a gradual rise during sub-maximal exercise with a marked rise in peak exercise mPAP.
. Metabolic and hemodynamic data during CPET among the study patients. Results of the Cardiopulmonary exercise testing. All patients had maximal effort tests with RER > 1.1. VO2 is a breath-by breath measurement of oxygen consumption. VO2/kg percent predicted was determined using standardized equations8. VE/VCO2 slope reported as the ratio of minute ventilation (VE) to expired Carbon Dioxide (VCO2) from start of exercise to peak exercise.
. <b>Implanted hemodynamic monitor (CardioMEMs) measurements during exercise.</b> Data are presented for each patient at each phase of exercise. There is a graphical representation of the IHM measured PAp and the table below shows corresponding vital signs and exercise capacity. Anaerobic threshold (AT) was determined by the attending cardiologist. Note patients 1 and 3 have patent fenestrations. Patients 2 and 4 were on pulmonary vasodilator therapy at the time of exercise.
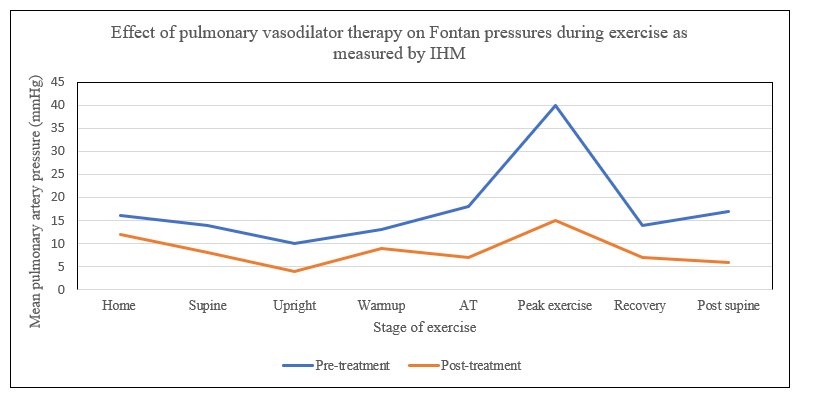
One patient was started on a phosphodieasterase-5 inhibitor for clinical reasons after the catheterization and CPET. The patient returned about 6 months later for a follow up CPET (). The follow up study showed that this patient had lower baseline mPAP and her mPAP remained low throughout exercise with the most notable change at peak exercise (40 mmHg vs. 15 mmHg). Exercise capacity at ventilatory anerobic threshold was higher (19.3 mL/kg/min vs. 20.3 mL/kg/min) and ventilatory efficiency slope had improved (40.5 vs. 36.3) suggesting better ventilation-perfusion matching. Oxygen Saturations were higher at rest (96% vs. 85%) as well as at peak exercise (87% vs. 71%). All of these changes indicated improvement in Fontan hemodynamics with medical therapy.
. <b>Implanted hemodynamic monitor (CardioMEMs) measurements before and after initiation of pulmonary vasodilator therapy.</b> Serial recordings were obtained during exercise using IHM on patient 1, before and after commencing pulmonary vasodilator therapy with Phosphodiesterase-5 inhibitor (PDE5i). The graph shows decrease in Fontan pressure at baseline and all stages of exercise with the most profound difference seen at peak exercise showing a reduction from 40 mmHg to 15 mmHg.
3. Discussion
We demonstrate the utility of IHM (CardioMEMs device) in the assessment of mPAP during exercise in patients with Fontan circulation. Exercise is critical in understanding Fontan circulation and in prognosis of outcome in Fontan circulation. Prior to utilization of IHM, invasive procedures were required to directly measure or estimate mPAP. Our institutional practice is to place the IHM at the time of routine cardiac catheterization for patients with failing Fontan physiology thus not adding additional invasive procedures or morbidity [
2]. In addition to mPAP monitoring during exercise the IHM also allows for medical management with direct monitoring of mPAP outside the cath lab. There are other methods to measure mPAP during exercise [
10] but pediatric patients would not be tolerant of such invasive measures.
There are few studies reporting experience with IHM use in Fontan circulation, one series reported higher mPAP during exercise in patients with single morphological right ventricles [
7]. Our case series is small and with only one patient with a single morphological left ventricle, therefore it is challenging to make any conclusions. Although the IHM does provide insight about the mPAP it does not provide additional diagnostic data to explain why the mPAP may be abnormal. For example, Aortopulmonary collaterals may provide a volume load into the pulmonary circulation and cause an increase in mPAP. The same change can be seen from a rising pulmonary artery wedge pressure which may also result in lower exercise capacity [
10]. Expertise with Fontan physiology and IHM characteristics will be critical in the interpretation of the mPAP data.
There are technical challenges in maintaining signal quality to obtain high fidelity measurements and a specialist who understands Fontan circulation is required to interpret IHM tracings. This is an off-label use so the IHM has been designed for a pulsatile system though it can be accurate with high fidelity measurements if utilized appropraitely. One key is to maintain a high signal strength connection which we achieved on an upright cycle ergometer. A recumbent cycle ergometer could also be utilized for a stress device though treadmill-based testing will not be possible. Even with cycle ergometer testing, interrogation of the IHM continuously during exercise requires several staff members. Apart from the feasability of measuring mPAP during exercise there is little in the literature about specifics of interpretation. Despite efforts to maintain high fidelity measurements we did have a seeming low supine pressure in patient 2 during the supine phase which was out of proportion to their home mPAP. This may have been related to hydration status or a fault with that particular measurement. There are reports of IHM requiring re-calibration [
8], however, our patients all performed CPET within a year of their IHM implantation and apart from this single data point the remainder of exercise and home readings have been appropriate.
One of our subjects had a peak exercise mPAP of 40 mmHg. This data contributed to the decision to initiate pulmonary vasodilator therapy. We demonstrated that IHM can show changes in mPAP over time after initiation of pulmonary vasodilator therapy. The change in peak exercise mPAP (drop by 25 mmHg) was far more substantial than the changes in her baseline mPAP (drop by 4 mmHg). There have been trials of initiation of pulmonary vasodilators in all patients [
11] with Fontan circulation however, as IHM utilization increases this creates an opportunity for goal-directed therapy for individual patients. Further study of this device may reveal the clinical implications of the Fontan pressures measured during CPET.
4. Learning Objectives
To understand how an implanted hemodynamic monitoring device may be utilized to evaluate Fontan pressures during a cardiopulmonary exercise test.
Author Contributions
The study was conceptualized by A.S. and D.B., Investigation and Data Curation was performed by A.S., J.M., B.C and D.B. Manuscript was written by A.S. and D.B., Review and editing was performed by A.S., J.M., B.C. and D.B.
Ethics Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Phoenix Children’s Hospital
Informed Consent Statement
This report features off label use in pediatric patients.
Funding
This research received no external funding.
Declaration of Competing Interest
The authors have no financial disclosures or other relationships.
References
1.
Rychik J, Atz AM, Celermajer DS, Deal BJ, Gatzoulis MA, Gewillig MH, et al. American Heart Association Council on Cardiovascular Disease in the Young and Council on Cardiovascular and Stroke Nursing. Evaluation and Manage-ment of the Child and Adult with Fontan Circulation: A Scientific Statement from the American Heart Association.
Circulation 2019,
140, e234–e284. doi:10.1161/CIR.0000000000000696.
[Google Scholar]
2.
Bhat DP, Graziano JN, Garn BJ, Franklin WJ. Safety and utility of CardioMEMS device for remote pulmonary artery monitoring in paediatric Fontan patients: a case series.
Eur. Heart J. Case Rep. 2023,
7, ytad422. doi:10.1093/ehjcr/ytad422.
[Google Scholar]
3.
Weinreb SJ, Dodds KM, Burstein DS, Huang J, Rand EB, Mancilla E, et al. End-Organ Function and Exercise Perfor-mance in Patients with Fontan Circulation: What Characterizes the High Performers?
J. Am. Heart Assoc. 2020,
9, e016850. doi:10.1161/JAHA.120.016850.
[Google Scholar]
4.
Cooper DM, Weiler-Ravell D. Gas exchange response to exercise in children.
Am. Rev. Respir. Dis. 1984,
129 (2 Pt 2), S47–S48. doi:10.1164/arrd.1984.129.2P2.S47.
[Google Scholar]
5.
Miranda WR, Egbe AC, Van De Bruaene A, Connolly HM, Burchill LJ, Jain CC. Peripheral Venous Pressure Accu-rately Reflects Invasively Measured Resting and Exercise Fontan Pressures.
Am. J. Cardiol. 2024,
215, 62–64. doi:10.1016/j.amjcard.2024.01.014.
[Google Scholar]
6.
Khedraki R, Abraham J, Jonsson O, Bhatt K, Omar HR, Bennett M, et al. Impact of exercise on pulmonary artery pressure in patients with heart failure using an ambulatory pulmonary artery pressure monitor.
Front. Cardiovasc. Med. 2023,
10, 1077365. doi:10.3389/fcvm.2023.1077365.
[Google Scholar]
7.
Bradley EA, Jassal A, Moore-Clingenpeel M, Abraham WT, Berman D, Daniels CJ. Ambulatory Fontan pressure mon-itoring: Results from the implantable hemodynamic monitor Fontan feasibility cohort (IHM-FFC).
Int. J. Cardiol. 2019,
284, 22–27. doi:10.1016/j.ijcard.2018.10.081.
[Google Scholar]
8.
Marshall WH, 5th, Rajpal S, Mah ML, Armstrong AK, Salavitabar A, Hickey J, et al. Early Experience and Lessons Learned Using Implanted Hemodynamic Monitoring in Patients With Fontan Circulation.
J. Am. Heart Assoc. 2023,
12, e031836. doi:10.1161/JAHA.123.031836.
[Google Scholar]
9.
Braunschweig F, Linde C, Adamson PB, Magalski A, Erdmann E, Kjellstrom B, et al. Continuous central haemody-namic measurements during the six-minute walk test and daily life in patients with chronic heart failure.
Eur. J. Heart Fail. 2009,
11, 594–601. doi:10.1093/eurjhf/hfp045.
[Google Scholar]
10.
Miranda WR, Jain CC, Borlaug BA, Jaffe AS, Connolly HM, Burchill LJ, et al. Exercise Capacity, NT-proBNP, and Exercise Hemodynamics in Adults Post-Fontan.
J. Am. Coll. Cardiol. 2023,
81, 1590–1600. doi:10.1016/j.jacc.2023.02.031.
[Google Scholar]
11.
Goldberg DJ, Zak V, Goldstein BH, Schumacher KR, Rhode J, Penny DJ, et al. Results of the FUEL Trial. Circulation 2020, 141, 641–651; Erratum in Circulation 2020, 142, e31. doi:10.1161/CIRCULATIONAHA.119.044352.