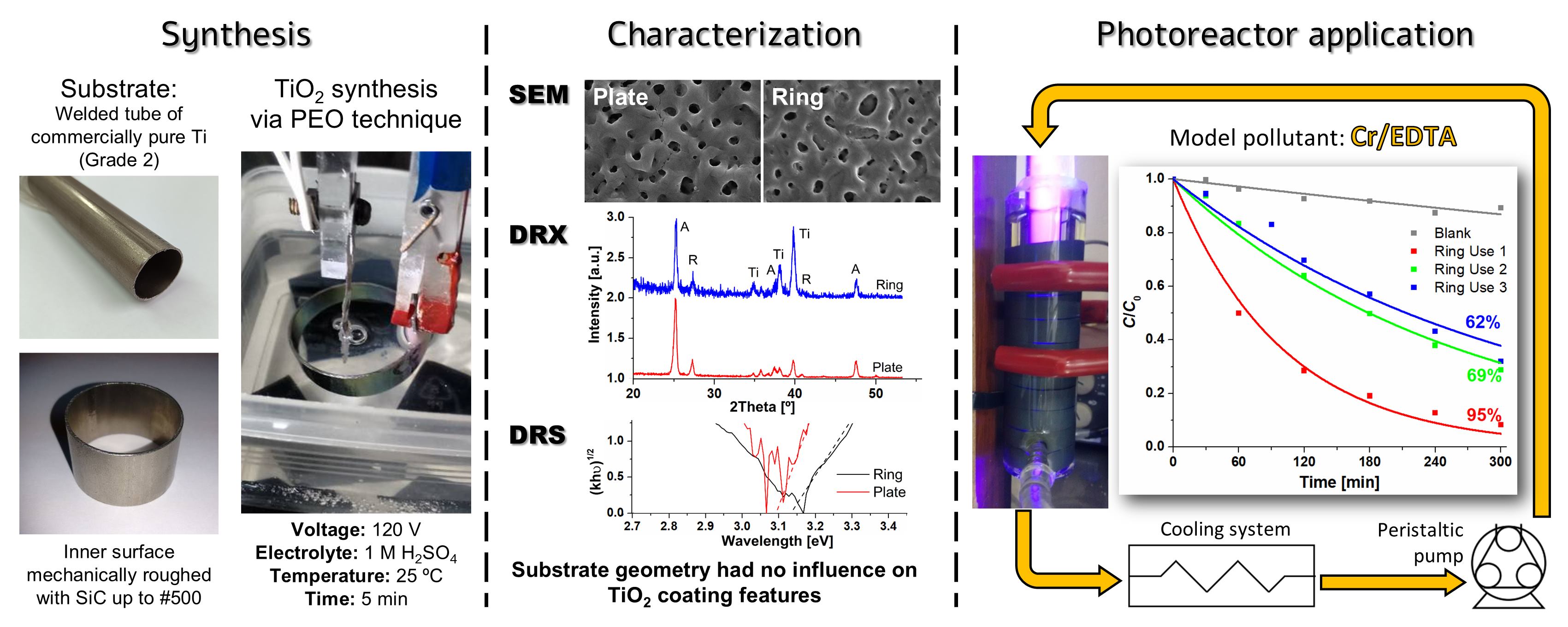
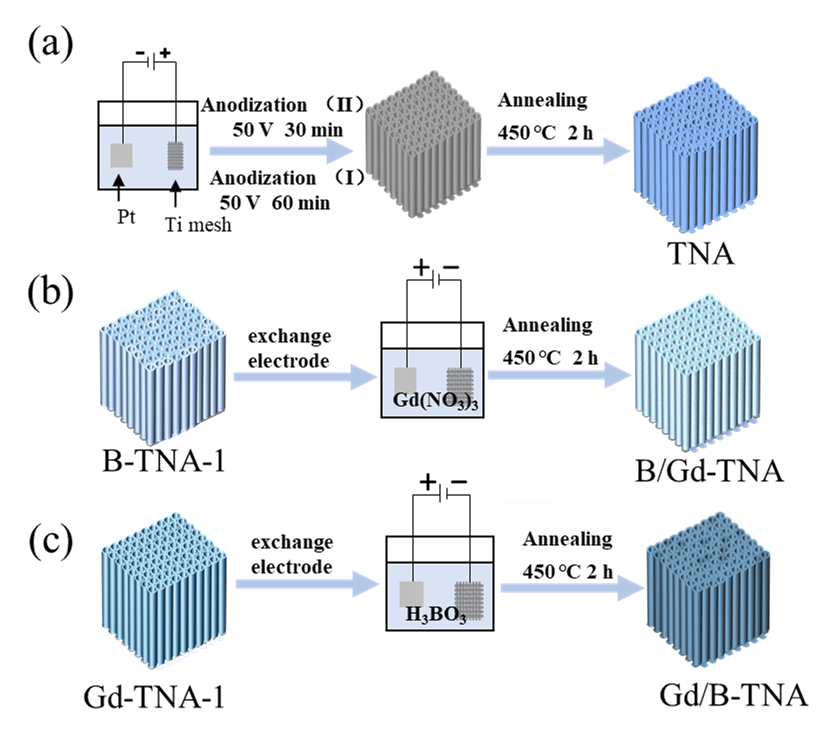
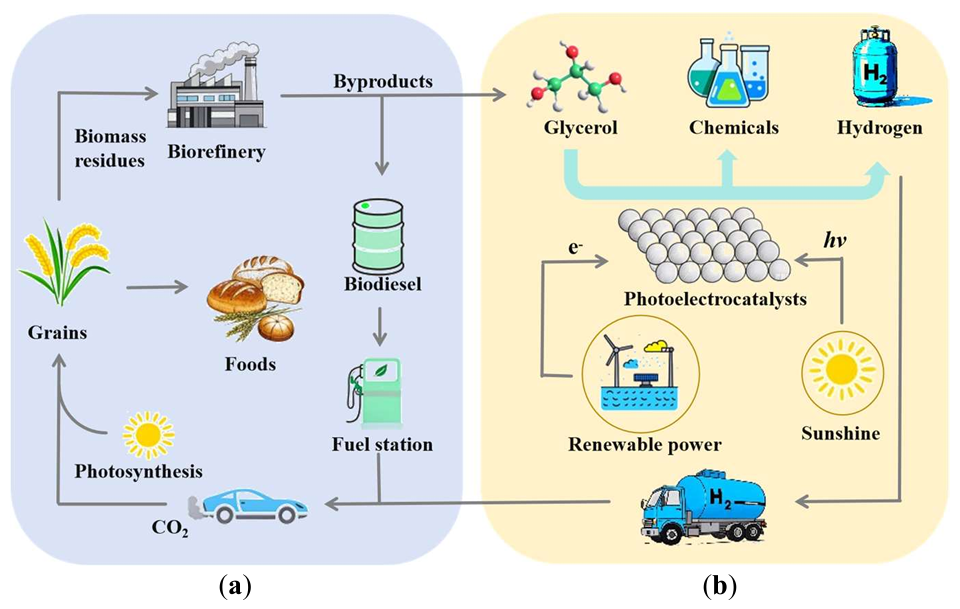
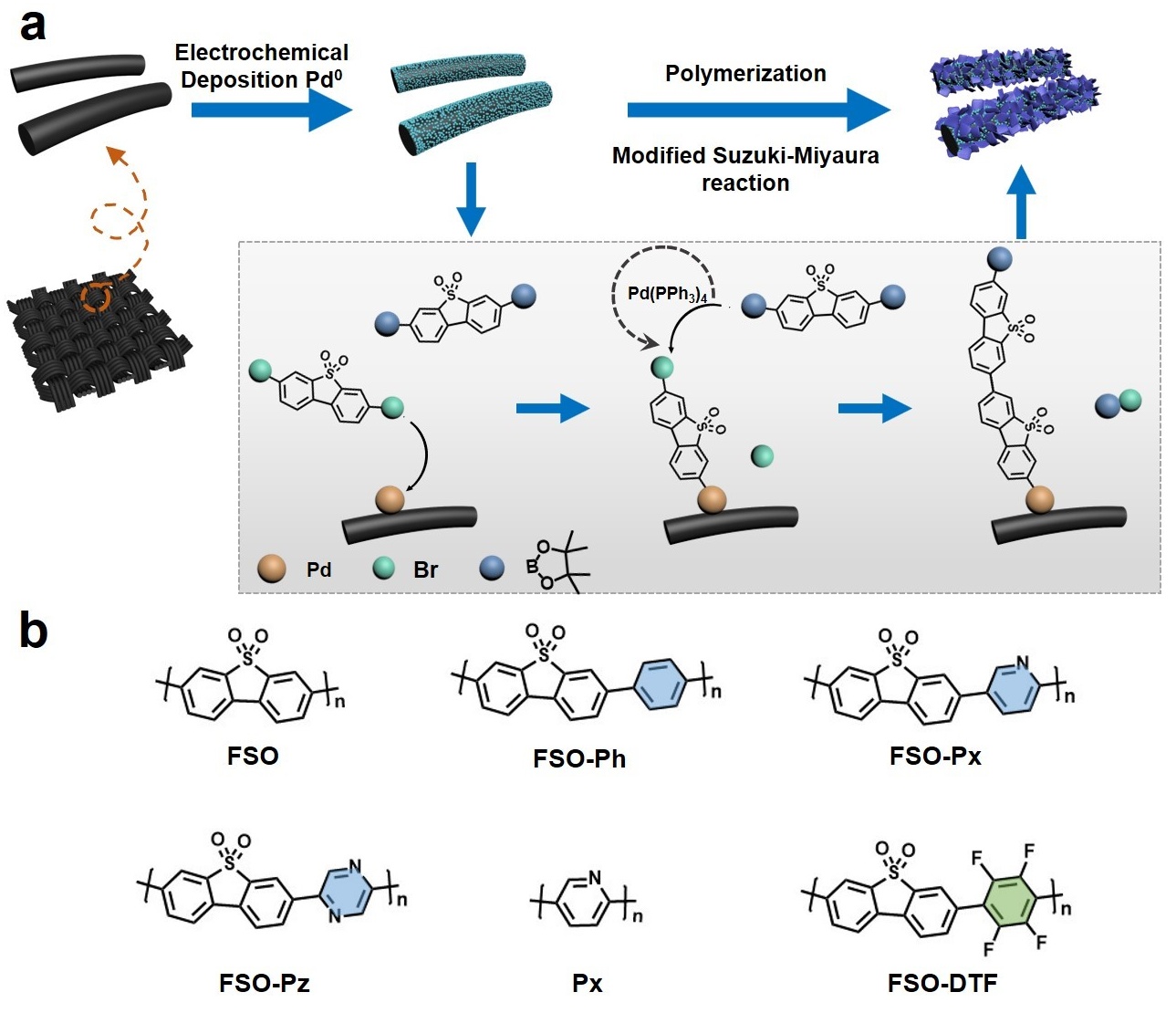
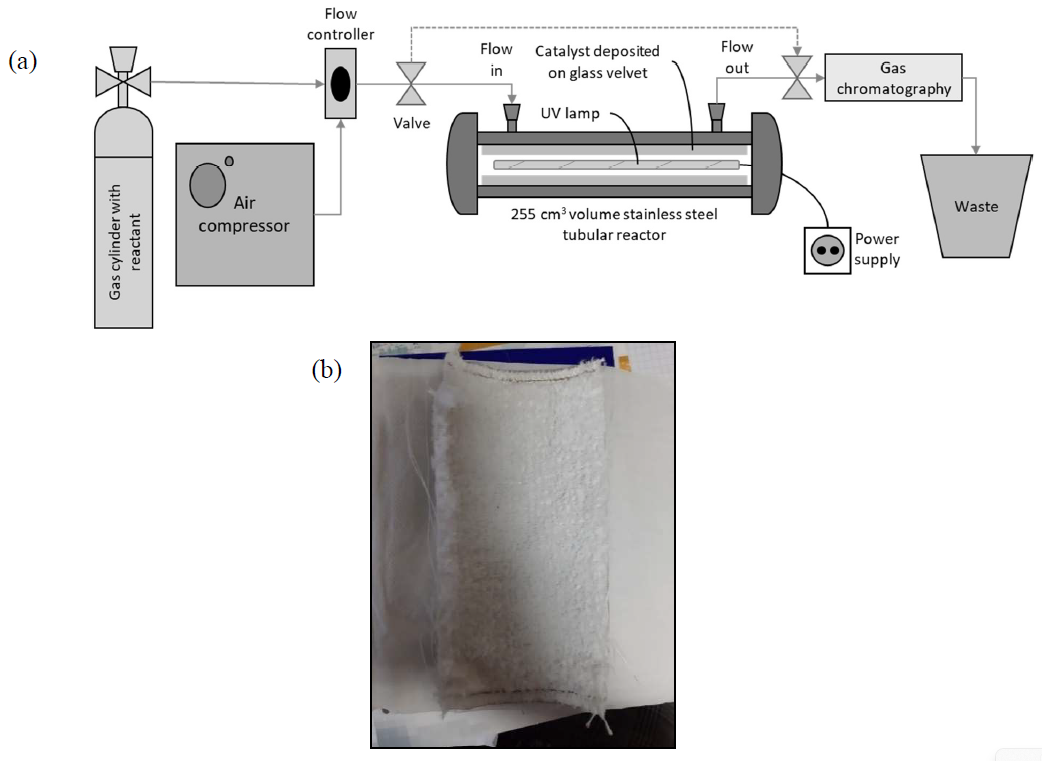
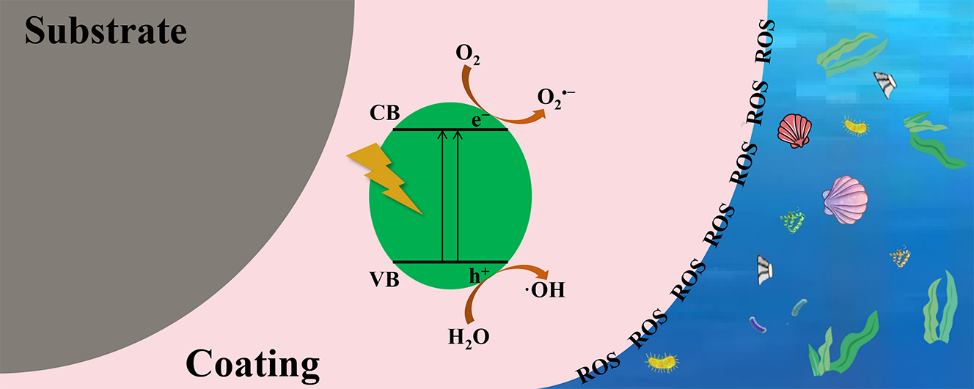
Photocatalysis: Research and Potential
 Open Access
Open Access
ISSN: 2958-8782 (Online)
2958-8774 (Print)
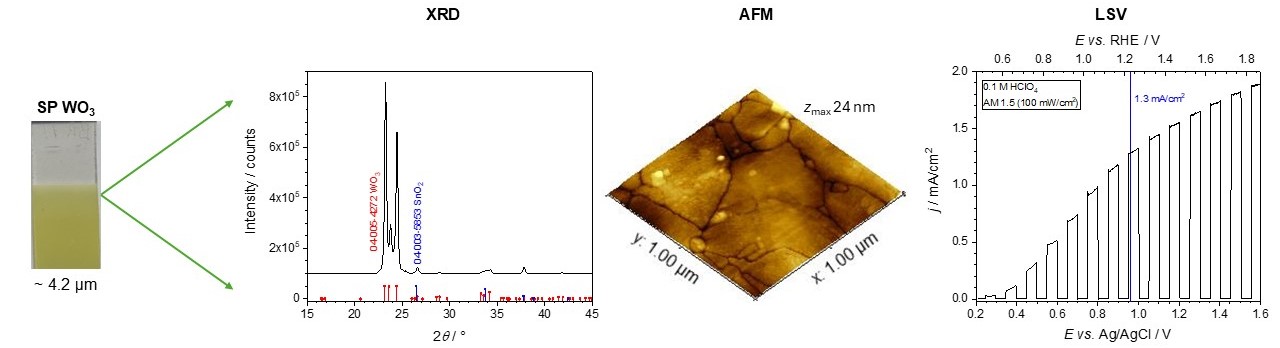
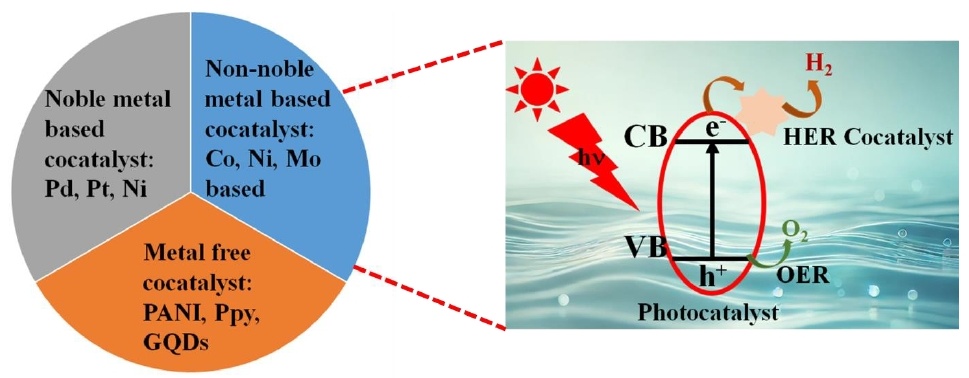
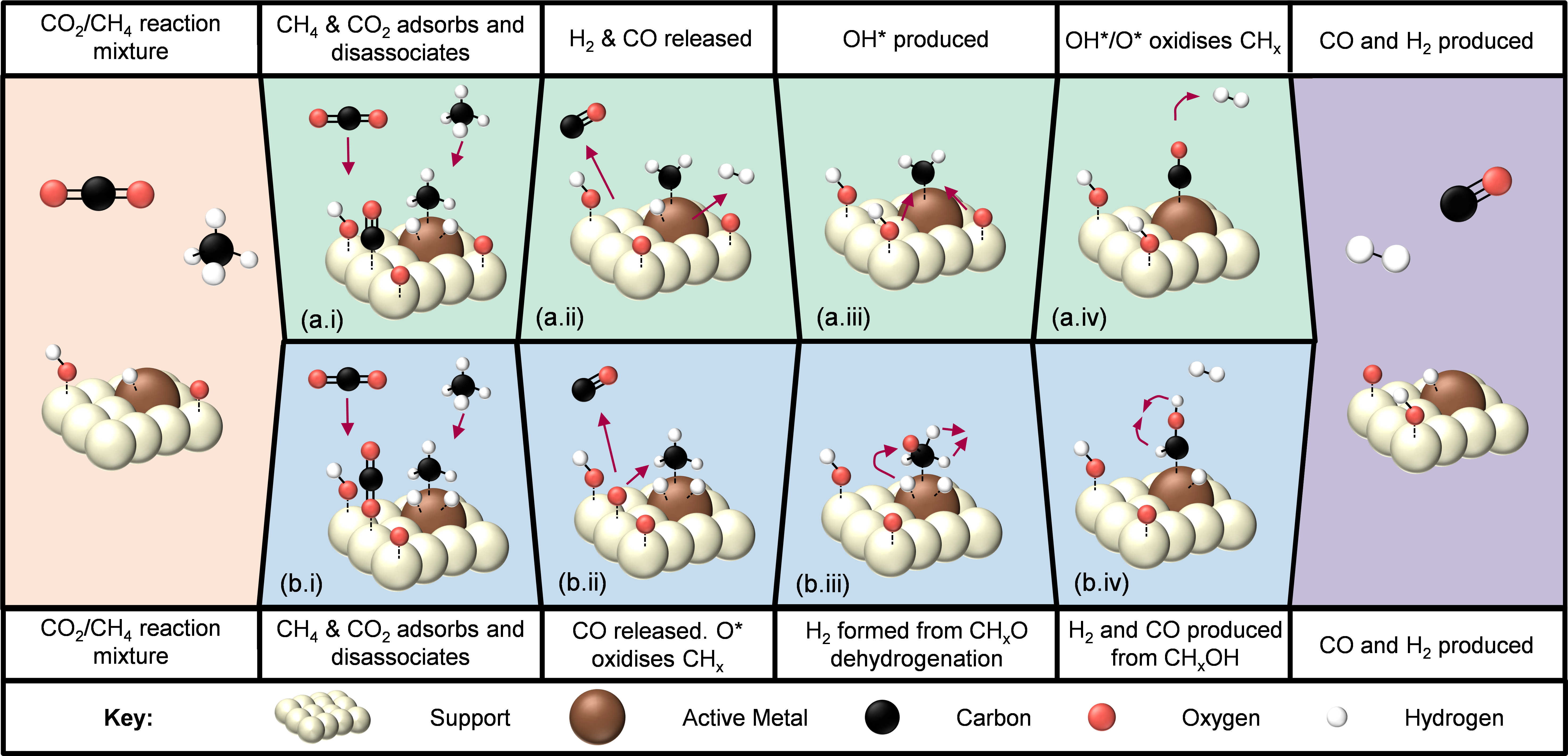
Photocatalysis: Research and Potential is an open access and international journal covering both fundamental and application-oriented aspects of photocatalysis. The journal establishes a platform for multidisciplinary discussions to promote the understanding of materials science, chemical engineering and physical chemistry of photocatalysis, as well as the potential across the field of environment and energy.