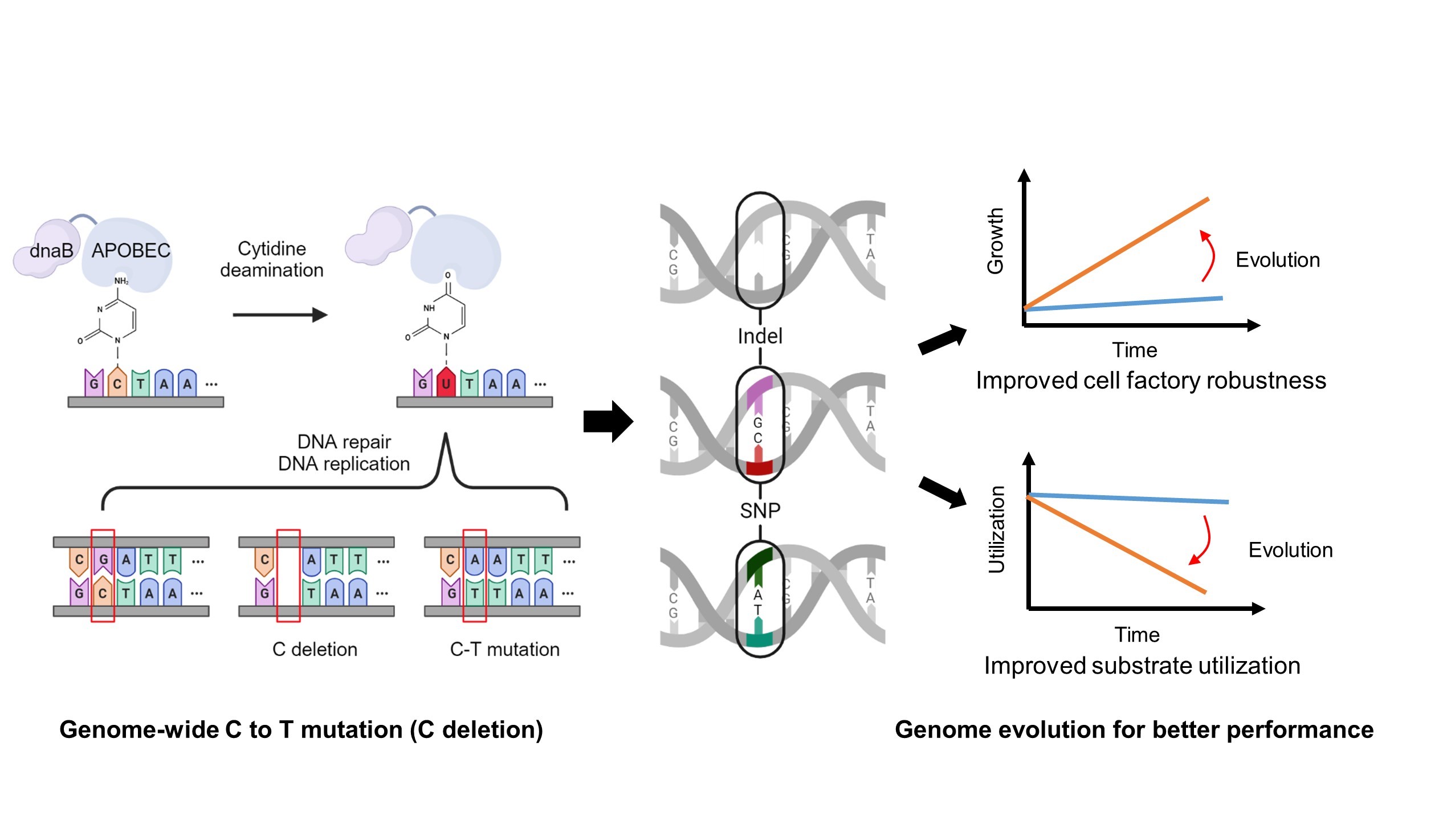
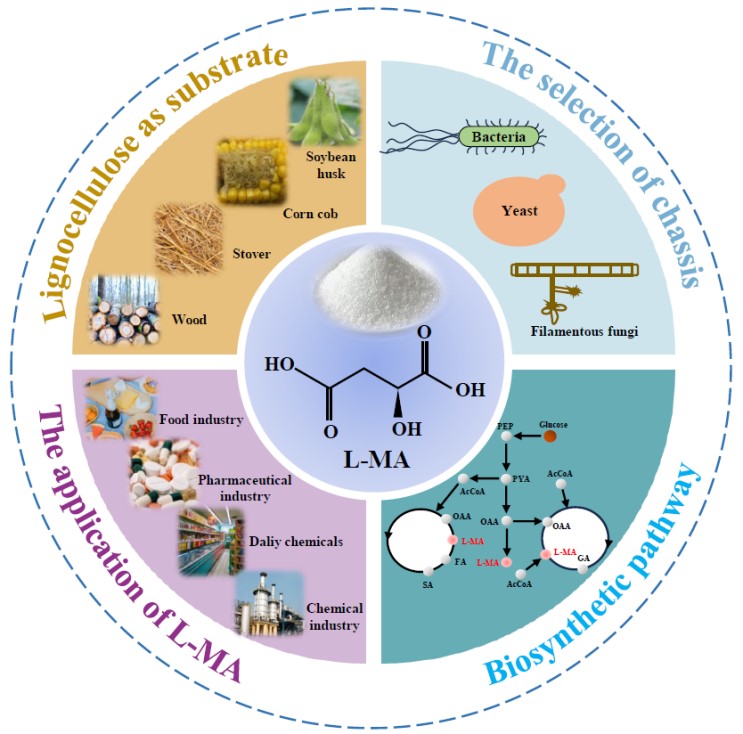
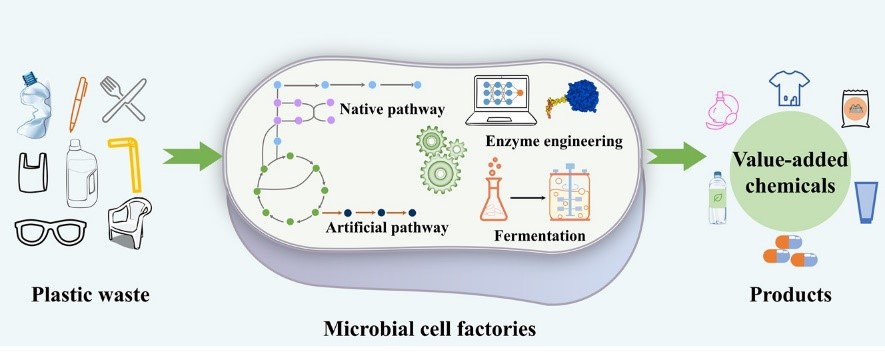
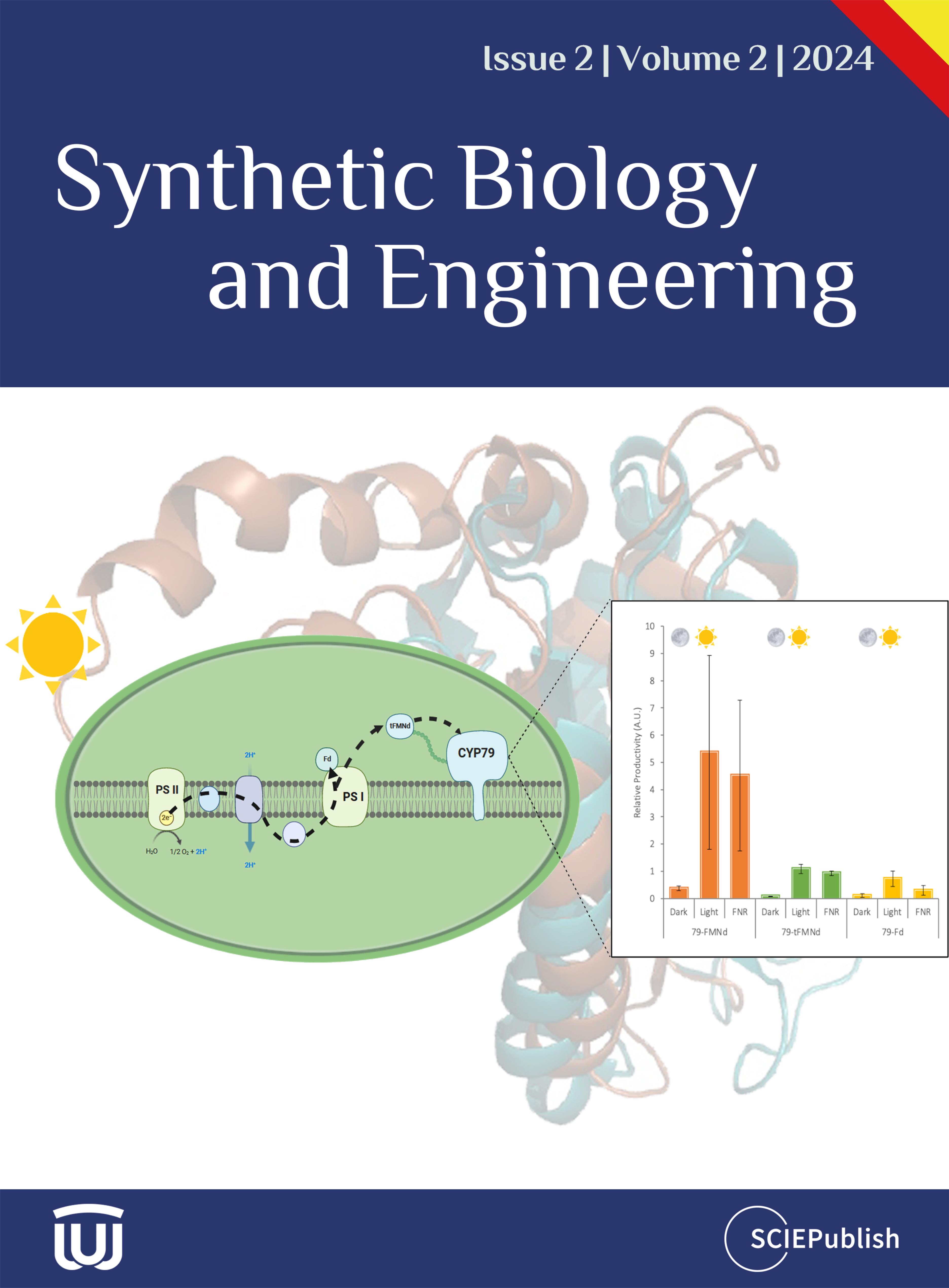
Great needs always motivate the birth and development of new disciplines and tools. Here we propose in vitro BioTransformation (ivBT) as a new biomanufacturing platform, between the two dominant platforms—microbial fermentation and enzymatic biocatalysis. ivBT mediated by in vitro synthetic enzymatic biosystems (ivSEBs) is an emerging biomanufacturing platform for the production of biocommodities (i.e., low-value and bulk biochemicals). ivSEB is the in vitro reconstruction of artificial (non-natural) enzymatic pathways with numerous natural enzymes, artificial enzymes, and/or (biomimetic or natural) coenzymes without living cell’s constraints, such as cell duplication, basic metabolisms, complicated regulation, bioenergetics, and so on. The two great needs (i.e., food security and the carbon-neutral renewable energy system) have motivated the birth and development of ivBT. Food security could be addressed by making artificial food from nonfood lignocellulose and artificial photosynthesis for starch synthesis from CO2. The carbon-neutral renewable energy system could be addressed by the construction of the electricity-hydrogen-carbohydrate cycle, where starch could be a high density of hydrogen carrier (up to 14.8% H2 wt/wt) and an electricity storage compound (greater than 3000 Wh/kg). Also, ivBT can make a number of biocommodities, such as inositol, healthy sweeteners (e.g., D-allulose, D-tagatose, D-mannose), advanced biofuels, polymer precursors, organic acids, and so on. The industrial biomanufacturing of the first several biocommodities (e.g., myo-inositol, D-tagatose, D-mannose, and cellulosic starch) would wipe off any prejudice and doubt on ivBT. Huge potential markets of biocommodities with more than tens of trillions of Chinese Yuan would motivate scientists and engineers to address the remaining technical challenges and develop new tools within the next decade.
 Open Access
Open Access