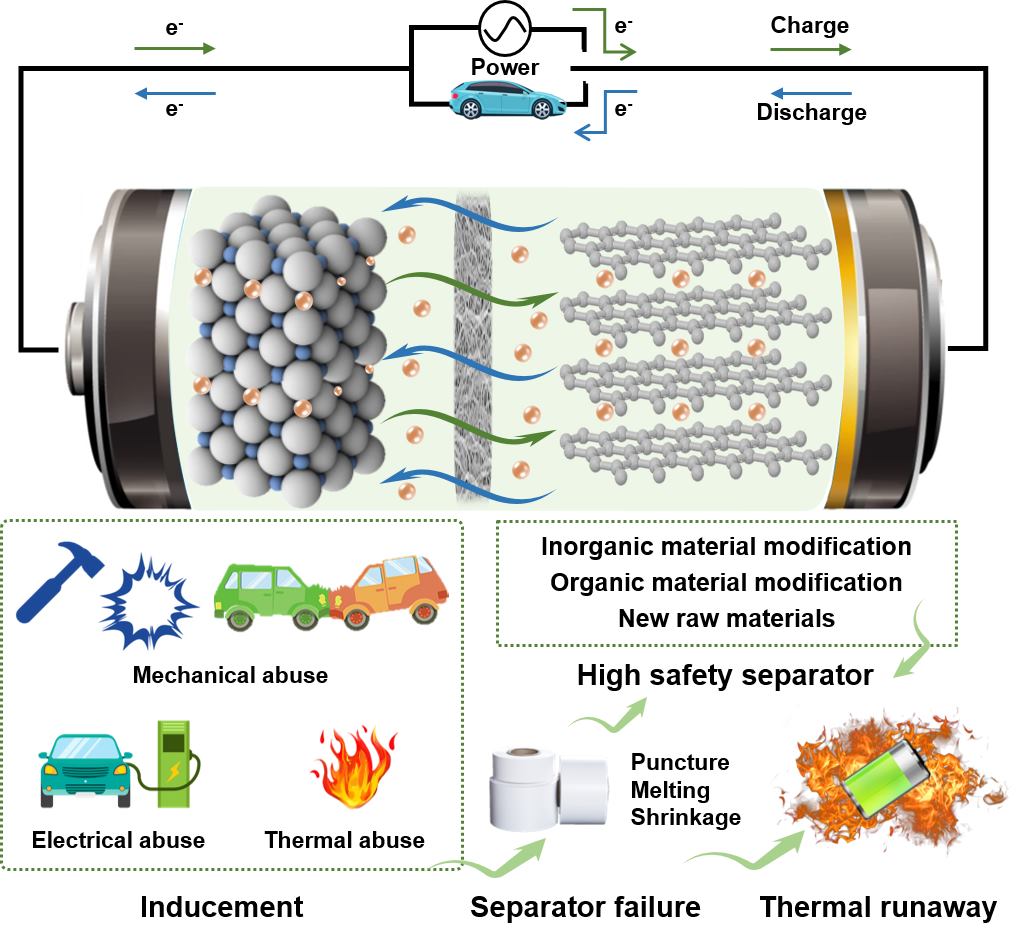
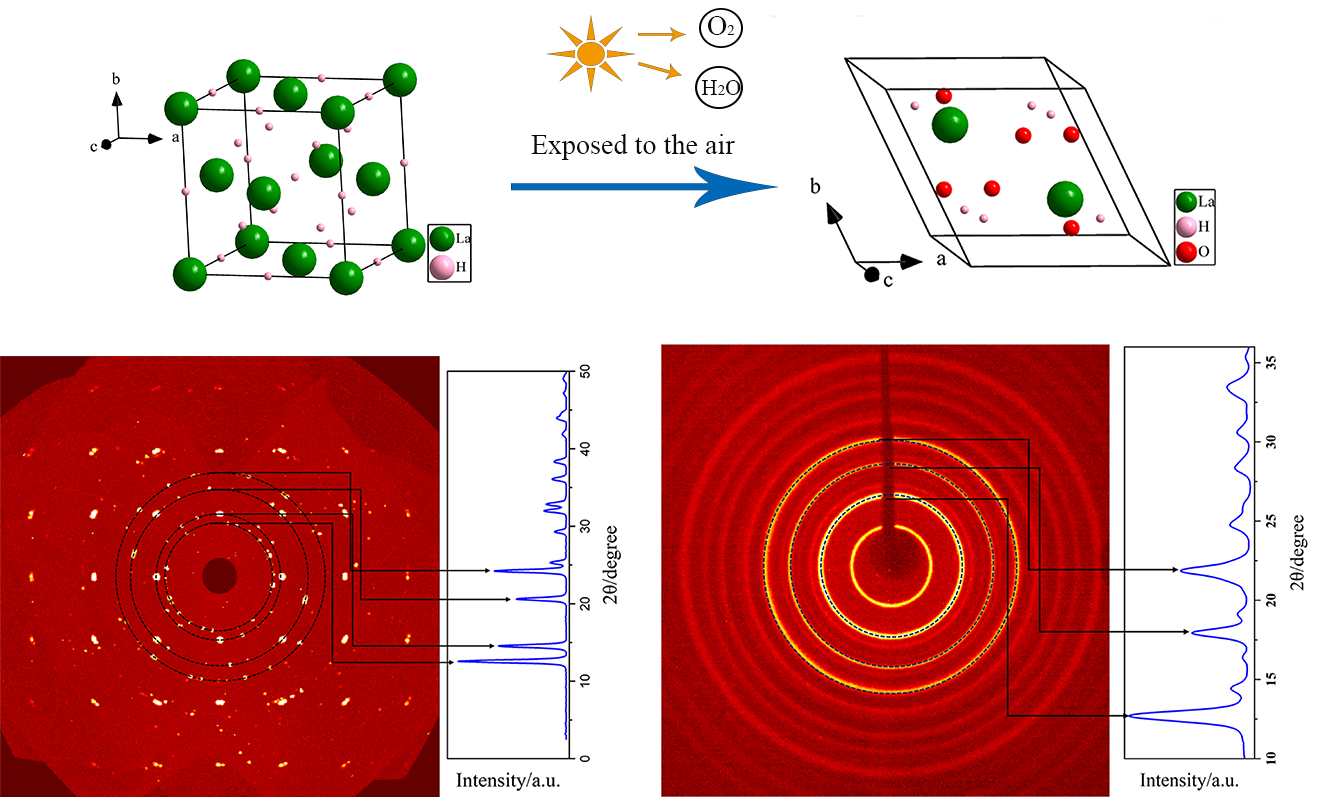
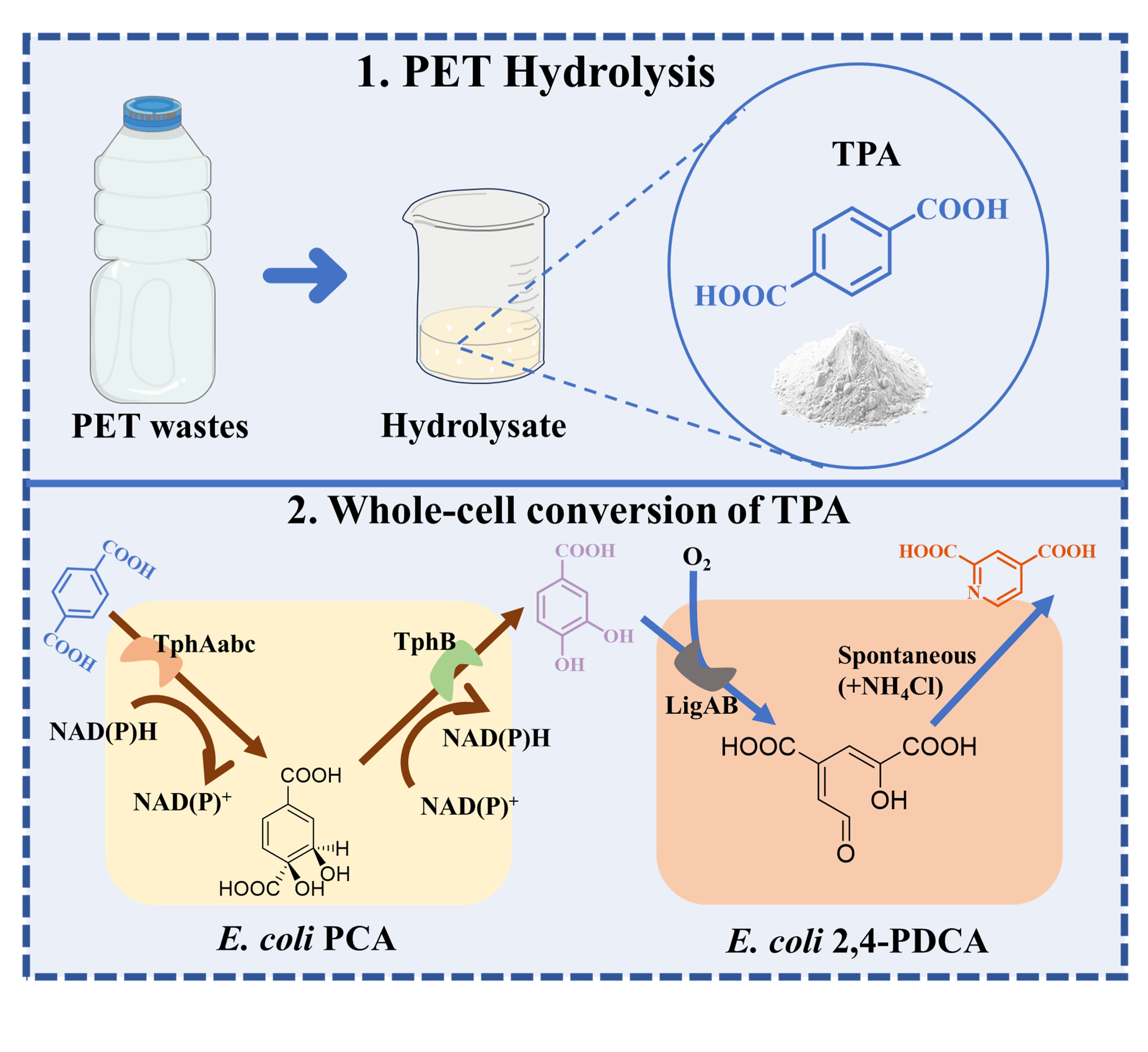
Green Chemical Technology
 Open Access
Open Access
ISSN: 3008-0886 (Online)
3008-0878 (Print)
Green Chemical Technology is an open access, peer-reviewed online journal that publishes articles on all aspects of green chemical technology: chemical engineering materials and process, mining and metallurgy development and technology, chemical synthesis, separation science, high-efficiency chemical equipment and intelligent control, and waste recycling. It is published quarterly online by SCIEPublish. View full Aims&Scope